filmov
tv
Hybridization of NH3 (ammonia)

Показать описание
NH3 has three single bonds to H's, and one lone pair.
NONE of that means there are pi bonds, and therefore there are no unhybridized p orbitals necessary.
Therefore, the hybridization is sp3.
NONE of that means there are pi bonds, and therefore there are no unhybridized p orbitals necessary.
Therefore, the hybridization is sp3.
Hybridization of NH3 (ammonia)
NH3 Hybridization: Hybrid Orbitals for NH3 (ammonia)
Hybridization of NH3 (Ammonia)
Orbital Overlap Diagram for NH3 (Ammonia)
Hybridisation of NH3 || SP3 hybridisation || Formation of ammonia molecule
NH3 hybridization || basic chemistry class || #shorts #saiclasses
Draw the Orbital Overlap Diagram for NH3 (ammonia)
sp3 Orbital Hybridization of Nitrogen in Ammonia NH3 - Organic Chemistry #stemeducation #stem #chem
Amines Class 12 | One Shot in English | JEE Main & Advanced
Ammonia hybridization l nh3 hybridization l chemistry l
Depicting Hybridization of Atomic Orbitals - Ammonia (NH3) 001
Ammonia NH3 Contour Diagram sp3 hybridization
What is the hybridization of NH3?
Hybridisation of NH3
This Is How We Do It... NH3 Hybridization with Elizabeth
Ammonia, ammonium and sp3 orbital hybridization
nitrogen hybridization/ sp3 hybridization in ammonia, orbital overlap diagram of nh3.
hybridization of NH3...
Hybridization of NH3 (Ammonia)
sp3 hybridization - quick build with organic chemistry model kit
hybridization of NH3 #chemistrybyms #shorts #viral #shortsvideo
Find the Hybridization of NH3?#chemistrytricks #neet2023 #neet #chemistryclass12tutorial #chemistry
hybridization of NH3 | hybridization of ammonia
SP3 Hybridisation of NH3 & H2O | chemical bonding | CBSE Class 11 Chemistry
Комментарии
 0:01:57
0:01:57
 0:00:44
0:00:44
 0:01:29
0:01:29
 0:06:13
0:06:13
 0:05:26
0:05:26
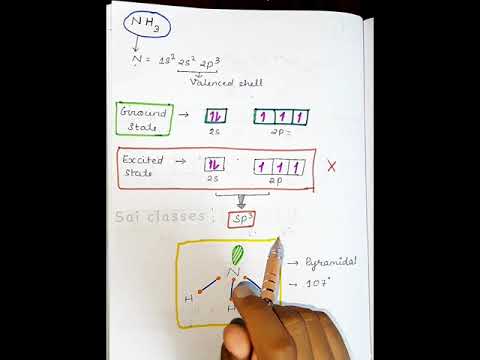 0:01:00
0:01:00
 0:07:07
0:07:07
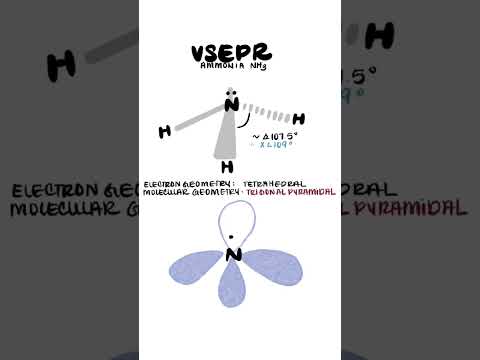 0:00:54
0:00:54
 2:53:26
2:53:26
 0:00:55
0:00:55
 0:05:33
0:05:33
 0:05:00
0:05:00
 0:02:39
0:02:39
 0:05:20
0:05:20
 0:02:28
0:02:28
 0:08:56
0:08:56
 0:10:46
0:10:46
 0:00:08
0:00:08
 0:01:29
0:01:29
 0:00:15
0:00:15
 0:00:16
0:00:16
 0:00:15
0:00:15
 0:03:13
0:03:13
 0:08:53
0:08:53