filmov
tv
Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3

Показать описание
This organic chemistry video tutorial explains the hybridization of atomic orbitals. It discusses how to determine the number of sigma and pi bonds in a molecule as well as determining if a carbon is sp, sp2, or sp3 hybridized. The full version of this video contains plenty of examples and practice problems.
Access The Full 36 Minute Video:
Direct Link to The Full Video:
Organic Chemistry PDF Worksheets:
___________________________________
Join The YouTube Membership Program:
Full 36 Minute Video on YouTube:
Access The Full 36 Minute Video:
Direct Link to The Full Video:
Organic Chemistry PDF Worksheets:
___________________________________
Join The YouTube Membership Program:
Full 36 Minute Video on YouTube:
Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3
Hybridization of Atomic Orbitals | SP, SP2, SP3 Hybridization of Carbon
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
EASY Method to Find the Hybridization of an Atom | Chemistry |
How to determine Hybridization - s, sp, sp2, and sp3 - Organic Chemistry
9.3 Hybridization | General Chemistry
Valence Bond Theory & Hybrid Atomic Orbitals
Orbitals: Crash Course Chemistry #25
DAY 44 | CHEMISTRY | II PUC | ALDEHYDES, KETONES AND CARBOXYLIC ACIDS | L2
Hybrid Orbitals explained - Valence Bond Theory | Orbital Hybridization sp3 sp2 sp
Sigma and Pi Bonds Explained, Basic Introduction, Chemistry
Hybridization of atomic orbitals. sp3d and sp3d2
Hybridization of atomic orbitals. sp2.
Hybridization of Atomic Orbitals
How to Determine the Hybridization of an Atom (sp, sp2, sp3, sp3d, sp3d2) Practice Problem & Exa...
14. Valence Bond Theory and Hybridization
14.2.2 Hybridisation of atomic orbitals
1.3 Valence Bond Theory and Hybridization | Organic Chemistry
Atomic Orbitals
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
Ch#3 | lec#4 | Hybridization of Atomic Orbitals | Rules for hybridization
Hybridization of atomic orbitals
Hybridization, Orbital Overlap, and Bond Length
Hybridization of Atomic Orbitals
Комментарии
 0:10:55
0:10:55
 0:13:48
0:13:48
 0:07:54
0:07:54
 0:04:08
0:04:08
 0:08:22
0:08:22
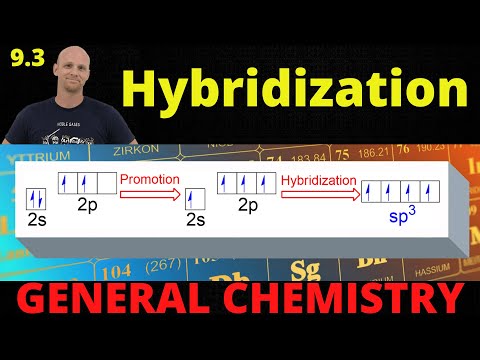 0:16:52
0:16:52
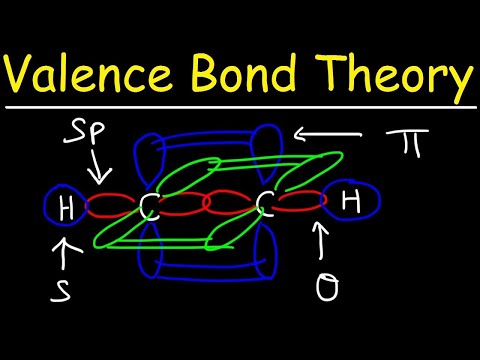 0:10:39
0:10:39
 0:10:52
0:10:52
 0:22:21
0:22:21
 0:11:58
0:11:58
 0:06:17
0:06:17
 0:08:02
0:08:02
 0:09:08
0:09:08
 0:11:50
0:11:50
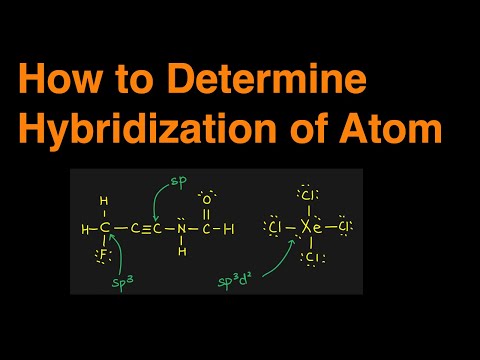 0:03:35
0:03:35
 0:56:46
0:56:46
 0:02:50
0:02:50
 0:26:04
0:26:04
 0:02:50
0:02:50
 0:11:19
0:11:19
 0:14:51
0:14:51
 0:10:23
0:10:23
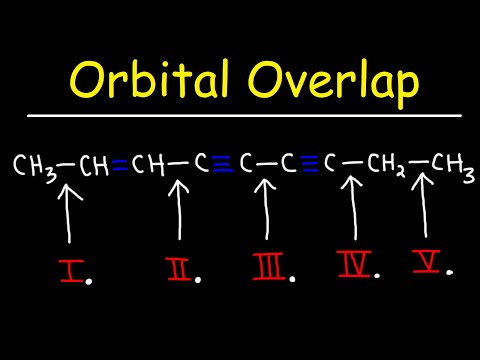 0:08:03
0:08:03
 0:49:52
0:49:52