filmov
tv
Predicting Organic Reaction Equilibrium From pKa Values

Показать описание
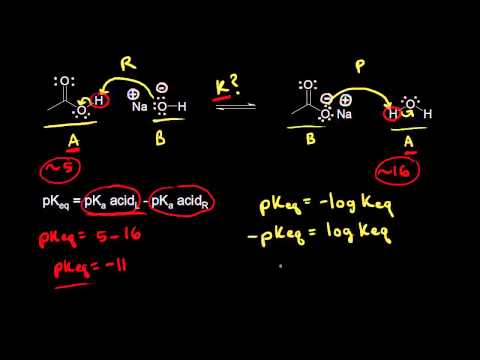
This lecture we focus on using the pKa values for various organic acids to predict which side of an organic reaction will be favored at equilibrium.
Support the Channel!
Private 1 on 1 Coaching Available, Custom Video Lessons!
Support the Channel!
Private 1 on 1 Coaching Available, Custom Video Lessons!
Predicting Organic Reaction Equilibrium From pKa Values
Predicting The Position of Equilibrium Using pKa values - Acids and Bases
Chemical Equilibria and Reaction Quotients
Using pKa values to predict the position of equilibrium | Organic chemistry | Khan Academy
Exercise 3.29 - Predicting the Position of Equilibrium without using pKa Values
How To Predict The Reactant and Reagent of Acid Base Reactions
Le Chatelier's Principle
GCSE Chemistry - Reversible Reactions and Equilibrium
Le Chatelier's Principle
Lecture on Predicting the Equilibrium Constant (Keq) for Acid-Base Reaction 001
A satisfying chemical reaction
Predict Equilibrium Position With Mayya
How to Predict Acid-Base Reactions from pKa Values Ft. Professor Dave
Predicting Precipitation With Ksp Values
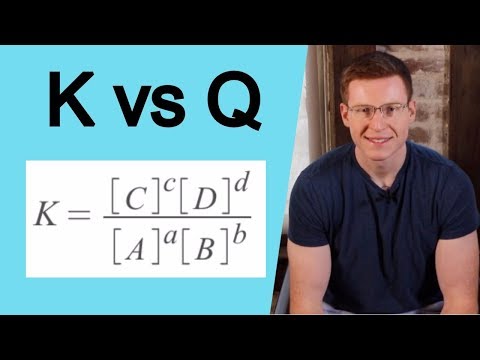
K (Equilibrium Constant) vs Q (Reaction Quotient)
Chemical Equilibrium: Predicting Acid-Base Reactions Using the Acid Table
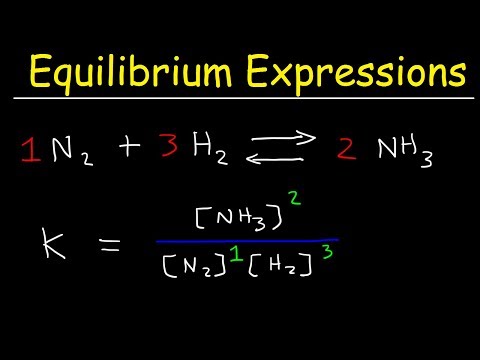
How To Write The Equilibrium Expression For a Chemical Reaction - Law of Mass Action
Predict the Products of Organic Reactions 002
Identifying the acid and base in an acid-base reaction #acidsandbases #organicchemistry
Determine Rate Law from Reaction Mechanisms, Fast then Slow Step: Part I
Hydrophobic Club Moss Spores
Predicting Products of Acid/Base Reactions
Chapter 14. Predicting the Direction of an Equilibrium Reaction Using the Reaction Quotient
Predicting The Products of Chemical Reactions - Chemistry Examples and Practice Problems
Комментарии
 0:12:36
0:12:36
 0:09:02
0:09:02
 0:06:48
0:06:48
 0:07:54
0:07:54
 0:09:51
0:09:51
 0:20:38
0:20:38
 0:04:09
0:04:09
 0:06:01
0:06:01
 0:26:40
0:26:40
 0:08:06
0:08:06
 0:00:19
0:00:19
 0:03:40
0:03:40
 0:02:42
0:02:42
 0:06:49
0:06:49
 0:03:00
0:03:00
 0:08:27
0:08:27
 0:05:24
0:05:24
 0:02:25
0:02:25
 0:00:34
0:00:34
 0:07:46
0:07:46
 0:00:31
0:00:31
 0:07:36
0:07:36
 0:16:22
0:16:22
 0:18:42
0:18:42