filmov
tv
Predicting Precipitation With Ksp Values

Показать описание
Now that we know about the solubility product, it's time to learn about some applications for this concept. First, we can use this to predict whether precipitation will occur in a particular solution, and take measures to cause or prevent precipitation, depending on the kind of chemistry we are interested in doing. Let's learn how to do this now!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Predicting Precipitation With Ksp Values
Worked example: Predicting whether a precipitate forms by comparing Q and Kₛₚ | Khan Academy
Chemistry 30 - Predicting Precipitation (ksp) example
Solubility Product Constant (Ksp)
Predicting precipitation when mixing 2 solutions
Solubility Product and Predicting Precipitation // HSC Chemistry
Chem 8.6 - Predicting Precipitates in a reactinon (Ksp)
Predicting Precipitation Q v Ksp
How to Determine if Precipitate will Form or Not Examples, Practice Problems, Qsp Ksp, Step by Step
The Common Ion Effect
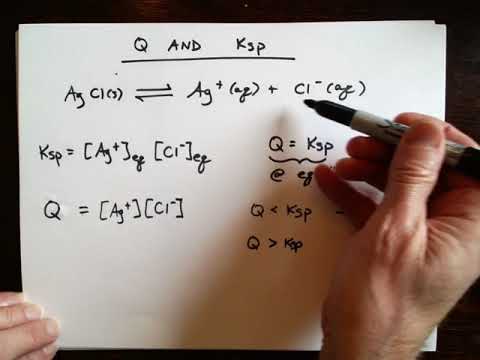
Chapter 17: Q, Ksp, and precipitation
predicting precipitation
Ksp - Molar Solubility, Ice Tables, & Common Ion Effect
Example: Determining Whether a Precipitate Will Form (Solubility Equilibrium #3)
⚗️ Predicting Precipitation Reactions by Comparing Q and Ksp
Predicting Precipitation I
Solubility Product Ksp Predicting Precipitation
Predicting Precipitation When Mixing Solutions
AP chem 11.4 - Ksp, Q, and dissolving precipitates
PREDICTING PRECIPITATION USING SOLUBILITY PRODUCT
Precipitation reactions and Solubility product Ksp
Predicting Precipitation Reactions by Comparing Q and Ksp-Practice Problems
Predicting Precipitation Reactions using Ksp
Equilibria: 12 - Predicting Precipitation
Комментарии
 0:06:49
0:06:49
 0:08:16
0:08:16
 0:06:13
0:06:13
 0:08:36
0:08:36
 0:05:48
0:05:48
 0:10:04
0:10:04
 0:11:36
0:11:36
 0:04:13
0:04:13
 0:07:31
0:07:31
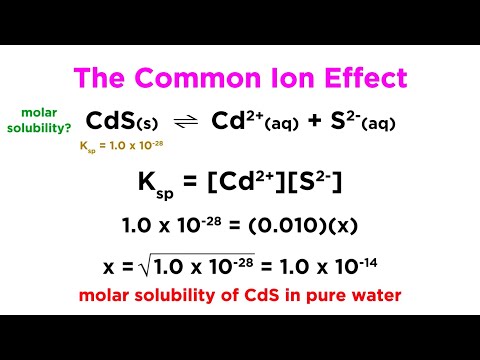 0:04:26
0:04:26
 0:04:26
0:04:26
 0:11:22
0:11:22
 0:41:52
0:41:52
 0:05:16
0:05:16
 0:03:44
0:03:44
 0:04:41
0:04:41
 0:34:03
0:34:03
 0:12:17
0:12:17
 0:16:40
0:16:40
 0:08:57
0:08:57
 0:06:47
0:06:47
 0:08:53
0:08:53
 0:08:29
0:08:29
 0:12:41
0:12:41