filmov
tv
Le Chatelier's Principle
Показать описание
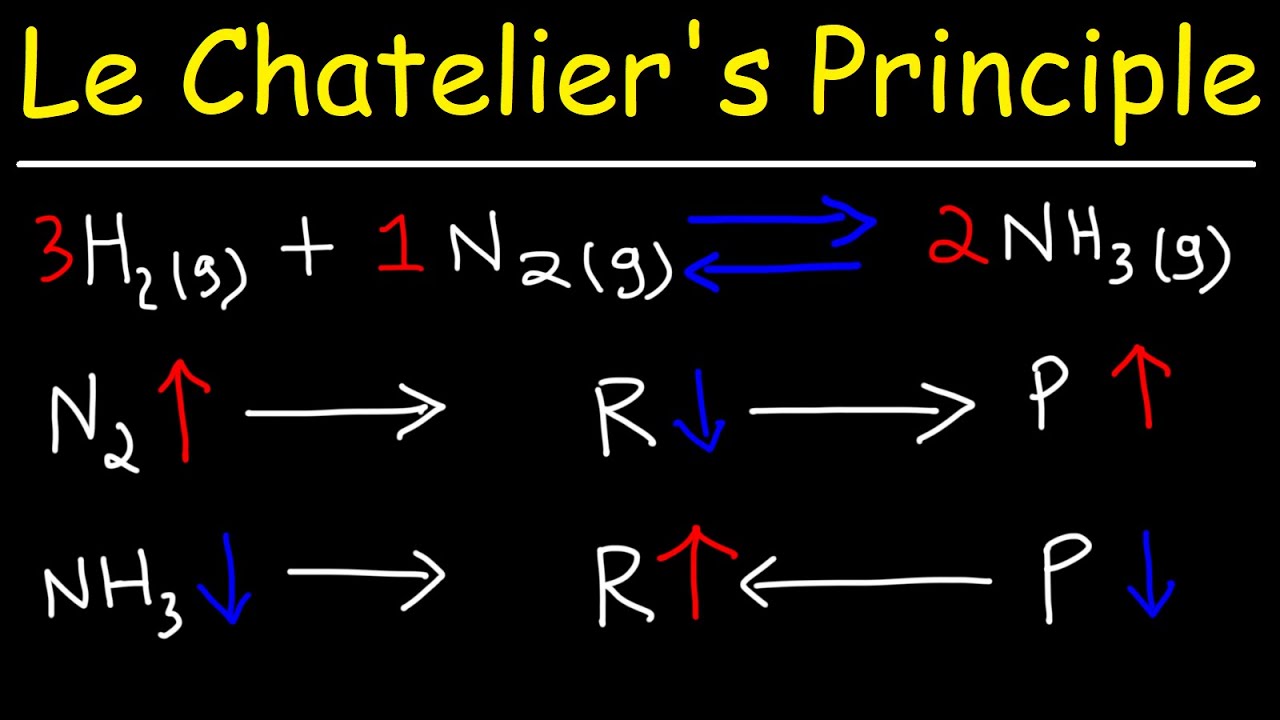
This chemistry video tutorial provides a basic introduction into Le Chatelier's Principle of chemical equilibrium. It explains how to determine which direction the reaction will shift if the concentrations of the reactants or the products increase in value. It discusses the effect of adding a catalyst or an inert gas on the position of equilibrium. It also discusses the effect of changing the volume and pressure on equilibrium.
Get The Full 1 Hour 10 Min Video:
PDF Worksheet With 7 Questions:
Direct Link to The Full Video:
Chemistry PDF Worksheets:
_____________________________
Full 1 Hour Video on YouTube:
Join The YouTube Membership Program:
Get The Full 1 Hour 10 Min Video:
PDF Worksheet With 7 Questions:
Direct Link to The Full Video:
Chemistry PDF Worksheets:
_____________________________
Full 1 Hour Video on YouTube:
Join The YouTube Membership Program:
Le Chatelier's Principle
GCSE Chemistry - Le Chatelier's Principle #50 (Higher Tier)
Le Chatelier's Principle
Le Chatelier Principle...in 5 minutes!
Le Chatelier's Principle | Effect of Concentration | Effect of Pressure | Effect of Temperature
Le Chatelier's Principle
Le Chatelier's Principle [IB Chemistry SL/HL]
Le Chȃtelier’s principle: Changing temperature | Equilibrium | AP Chemistry | Khan Academy
Le Châtelier's Principle: Equilibrium & Stress (Full Lesson) | Sketchy MCAT
Le Châtelier's principle | Reaction rates and equilibrium | High school chemistry | Khan Academ...
Le Chȃtelier’s principle: Changing concentration | Equilibrium | AP Chemistry | Khan Academy
Le Chatelier's Principle Part 1 | Reactions | Chemistry | FuseSchool
Le Chatelier's Principle grade 12: Introduction
Le Chȃtelier’s principle: Changing volume | Equilibrium | AP Chemistry | Khan Academy
LeChatelier Principle: Change Pressure
Le Chatelier's principle | University Of Surrey
Easy equilibrium – demonstrating Le Chatelier's principle
Le Chatelier's principle
CH13Q11 Le Chateliers Principle
Worked example: Using Le Chȃtelier’s principle to predict shifts in equilibrium | Khan Academy
9 06 Le Chatelier s Principle
Everything you need to know about Le Chateliers Principle (Exothermic, Endothermic, Pressure) MCAT
Le Chatelier's Principle and Temperature Changes (Pt. 10)
Le Chatelier Lab ANSWERS: Fe3+ and FeSCN2+ Equilibrium
Комментарии
 0:04:09
0:04:09
 0:03:51
0:03:51
 0:26:40
0:26:40
 0:05:50
0:05:50
 0:12:36
0:12:36
 0:07:01
0:07:01
 0:09:51
0:09:51
 0:11:16
0:11:16
 0:06:33
0:06:33
 0:05:46
0:05:46
 0:08:38
0:08:38
 0:04:15
0:04:15
 0:02:41
0:02:41
 0:11:09
0:11:09
 0:04:32
0:04:32
 0:02:02
0:02:02
 0:06:49
0:06:49
 0:07:51
0:07:51
 0:05:09
0:05:09
 0:03:58
0:03:58
 0:16:54
0:16:54
 0:13:03
0:13:03
 0:05:44
0:05:44
 0:06:28
0:06:28