filmov
tv
Predicting The Position of Equilibrium Using pKa values - Acids and Bases

Показать описание
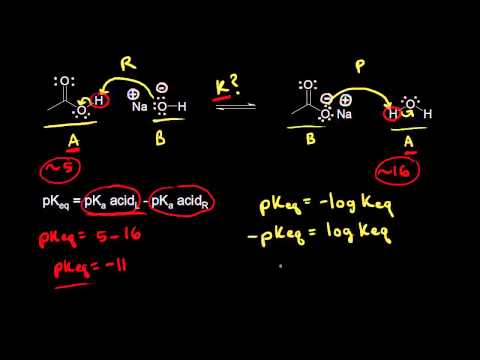
This organic chemistry video tutorial explains how to predict the products of an acid base reaction, how to draw the curve arrows to represent electron flow, how to identify the bronsted lowry acid and base as well as the conjugate acid and conjugate base, and finally how to predict the position of equilibrium using pKa values and determining if the reaction is product favored, reactant favored, or reversible.
Full 7 Hour Organic Chemistry Exam 1 Video:
Direct Link To The Full Video - Part 1:
Direct Link to The Full Video - Part 2:
PDF Worksheet - 90 Test Questions:
__________________________________
Exam 1 - Part 1 on YouTube:
Exam 1 - Part 2 on YouTube:
Organic Chemistry Exam 1 Playlist:
Organic Chemistry PDF Worksheets:
Full 7 Hour Organic Chemistry Exam 1 Video:
Direct Link To The Full Video - Part 1:
Direct Link to The Full Video - Part 2:
PDF Worksheet - 90 Test Questions:
__________________________________
Exam 1 - Part 1 on YouTube:
Exam 1 - Part 2 on YouTube:
Organic Chemistry Exam 1 Playlist:
Organic Chemistry PDF Worksheets:
Predicting The Position of Equilibrium Using pKa values - Acids and Bases
Exercise 3.29 - Predicting the Position of Equilibrium without using pKa Values
Using pKa values to predict the position of equilibrium | Organic chemistry | Khan Academy
What is a Position of Equilibrium?
Acid-base- ARIO 12, Predicting the position of the equilibrium- Dr. Tania CS
GCSE Chemistry - Le Chatelier's Principle (Higher Tier)
Le Chatelier's Principle
Le Chatelier's Principle
GCSE Chemistry - Reversible Reactions and Equilibrium
Position of Equilibrium and Choice of Reagents
Chemical Equilibria and Reaction Quotients
7.1 Equilibrium - predicting position of equilibrium
Equilibrium Unit Lesson 6: Predicting Reactions using the 5 step method
How to Determine What Side Acid-Base Equilibria Favor?
ALEKS: Using Le Chatelier’s Principle to predict the result of changing concentration
Le Chatelier's Principle | Effect of Concentration | Effect of Pressure | Effect of Temperature
Predict Direction of Acid Base Rxn
Acid-Base 6, predicting the position of the equilibrium using pKa- Dr. Tania CS
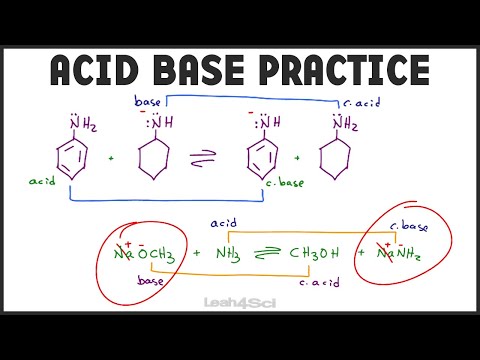
Acid Base Equilibrium Practice - Organic Chemistry
Acids and bases part3( predicting position of equilibrium)
5.20 predicting how the rate of attainment + equilibrium position is affected by changing conditions
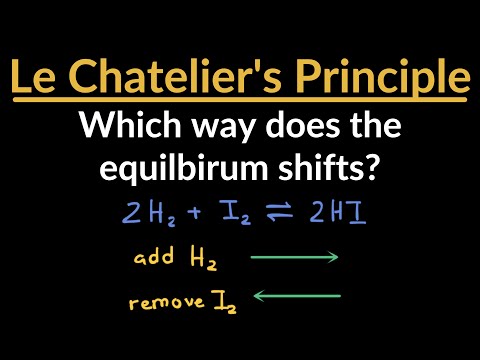
Which Direction Does the Reaction Shift / Le Châtelier’s Principle Practice Problems & Examples...
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
How To Predict The Reactant and Reagent of Acid Base Reactions
Комментарии
 0:09:02
0:09:02
 0:09:51
0:09:51
 0:07:54
0:07:54
 0:02:59
0:02:59
 0:06:54
0:06:54
 0:03:51
0:03:51
 0:04:09
0:04:09
 0:26:40
0:26:40
 0:06:01
0:06:01
 0:05:08
0:05:08
 0:06:48
0:06:48
 0:02:15
0:02:15
 0:23:42
0:23:42
 0:08:43
0:08:43
 0:03:14
0:03:14
 0:12:36
0:12:36
 0:09:10
0:09:10
 0:06:39
0:06:39
 0:11:07
0:11:07
 0:26:00
0:26:00
 0:03:18
0:03:18
 0:06:05
0:06:05
 0:53:22
0:53:22
 0:20:38
0:20:38