filmov
tv
Reaction Mechanisms, Rate Laws, Reaction Profiles, and SN1 vs. SN2 Reactions

Показать описание
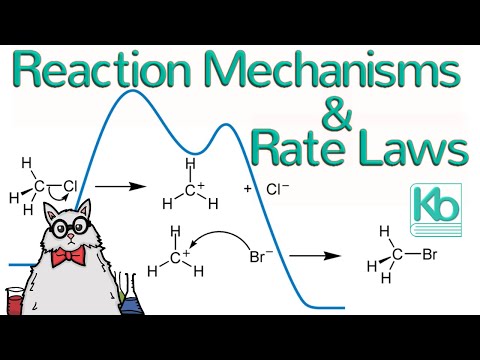
A reaction mechanism is a description of how a chemical reaction happens. The kinetics--and specifically the rate law--can be used to determine the mechanism of a reaction. In this video, we will look at a nucleophilic substitution reaction to better understand chemical kinetics.
chemical kinetics
rate law
reaction mechanism
reaction mechanism arrows
intermediate
transition state
reaction profile
reactants vs. products
exothermic vs. endothermic
enthalpy of reaction
activation energy
number of steps in a reaction
slow step = rate-determining step
SN1 vs. SN2
chemical kinetics
rate law
reaction mechanism
reaction mechanism arrows
intermediate
transition state
reaction profile
reactants vs. products
exothermic vs. endothermic
enthalpy of reaction
activation energy
number of steps in a reaction
slow step = rate-determining step
SN1 vs. SN2
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step - Chemical Kinetics
Reaction mechanism and rate law | Kinetics | AP Chemistry | Khan Academy
Determine Rate Law from Reaction Mechanisms, Fast then Slow Step: Part I
Kinetics: Initial Rates and Integrated Rate Laws
Reaction Mechanisms and rate laws: elementary steps and overall reaction
ECHE 430 - Lecture 18 - Rate Laws and Reaction Mechanisms
Rate Law For Mechanism With Fast Initial Step
Using Reaction Mechanisms (Rate Limiting Step) to Predict Rate Laws | Chemical Kinetics
A Level | Live Class 21 | Chemical Equilibria | Past Papers Solved | WhatsApp +92 323 509 4443
R2.2.6 Reaction mechanism, order of reaction and rate-determining step [HL IB Chemistry]
14.3 Reaction Mechanisms, Catalysts, and Reaction Coordinate Diagrams | General Chemistry
Reaction Mechanisms
Reaction Mechanisms with a Fast Initial Step Guided Practice
Reaction Mechanisms part 2: Finding Rate Laws
Reaction Mechanisms, Rate Laws, Reaction Profiles, and SN1 vs. SN2 Reactions
How to Find the Rate Law from Reaction mechanisms (and Graphically from Concentration Data)
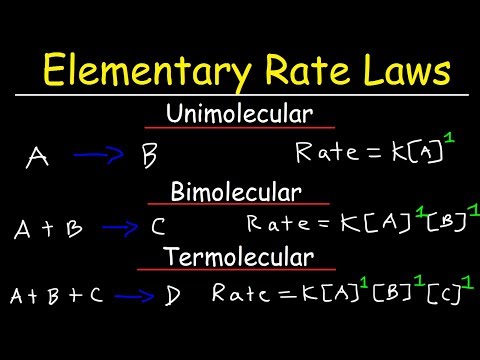
Elementary Rate Laws - Unimolecular, Bimolecular and Termolecular Reactions - Chemical Kinetics
32. Kinetics: Reaction Mechanisms
R2.2.9 Reaction mechanisms (HL)
Energy Diagrams, Catalysts, and Reaction Mechanisms
Integrated Rate Laws - Zero, First, & Second Order Reactions - Chemical Kinetics
KAC25.15 - Rates II: Proposing Reaction Mechanisms from Rate Equations
Rate law and reaction order | Kinetics | AP Chemistry | Khan Academy
[ASMR] How to write a rate law from a reaction mechanism
Комментарии
 0:18:48
0:18:48
 0:08:42
0:08:42
 0:07:46
0:07:46
 0:09:10
0:09:10
 0:06:23
0:06:23
 0:49:25
0:49:25
 0:02:49
0:02:49
 0:29:16
0:29:16
 0:54:56
0:54:56
 0:09:02
0:09:02
 0:36:56
0:36:56
 0:04:12
0:04:12
 0:03:37
0:03:37
 0:09:21
0:09:21
 0:18:44
0:18:44
 0:13:44
0:13:44
 0:04:28
0:04:28
 0:46:28
0:46:28
 0:10:01
0:10:01
 0:05:23
0:05:23
 0:48:46
0:48:46
 0:12:22
0:12:22
 0:10:39
0:10:39
![[ASMR] How to](https://i.ytimg.com/vi/vT3pDM7ha5U/hqdefault.jpg) 0:32:05
0:32:05