filmov
tv
Rate law and reaction order | Kinetics | AP Chemistry | Khan Academy

Показать описание
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Kinetics: Initial Rates and Integrated Rate Laws
Reaction Order Tricks & How to Quickly Find the Rate Law
Rate law and reaction order | Kinetics | AP Chemistry | Khan Academy
Integrated Rate Laws - Zero, First, & Second Order Reactions - Chemical Kinetics
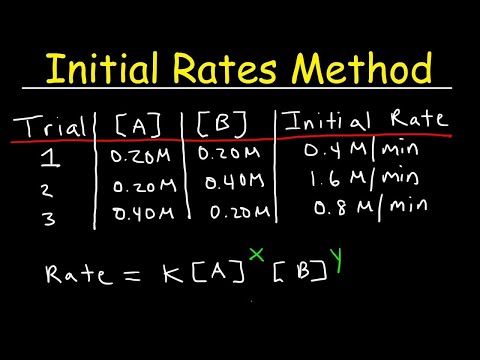
Chemical Kinetics - Initial Rates Method
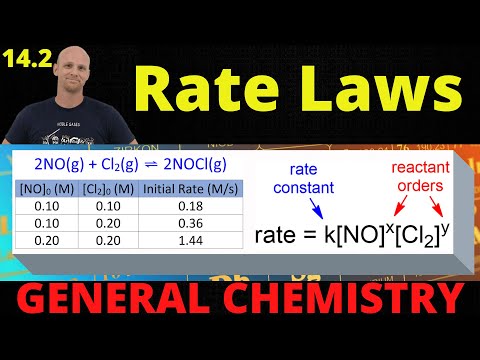
14.2 Rate Laws | General Chemistry
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step - Chemical Kinetics
How to Find the Rate Law and Rate Constant (k)
CHEMICAL KINETICS -2
The Rate Law
Rate Laws and Reaction Order
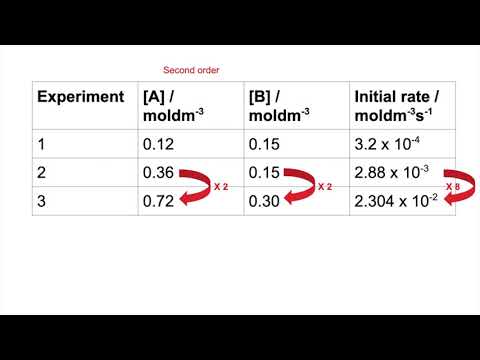
Working out order from a rate table - tricky example
How to Write a Rate Law
Solving a Rate Law Using the Initial Rates Method
Worked example: Determining a rate law using initial rates data | AP Chemistry | Khan Academy
14.2 Rate Laws
13.2 The Rate Law
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32
Determining Rate Laws from Experimental Data
DON'T MISS THIS Rate Law and Rate Constant Question
Order of Reaction
Determining Reaction Order From Rate Law 001
Reaction mechanism and rate law | Kinetics | AP Chemistry | Khan Academy
Kinetics: Using the Integrated Rate Laws and Graphs to Determine the Rate Law
Комментарии
 0:09:10
0:09:10
 0:01:58
0:01:58
 0:10:39
0:10:39
 0:48:46
0:48:46
 0:34:53
0:34:53
 0:25:16
0:25:16
 0:18:48
0:18:48
 0:03:42
0:03:42
 0:38:07
0:38:07
 0:08:44
0:08:44
 0:04:48
0:04:48
 0:02:46
0:02:46
 0:01:30
0:01:30
 0:10:49
0:10:49
 0:12:28
0:12:28
 0:19:43
0:19:43
 0:14:31
0:14:31
 0:09:57
0:09:57
 0:21:17
0:21:17
 0:03:46
0:03:46
 0:05:45
0:05:45
 0:02:43
0:02:43
 0:08:42
0:08:42
 0:09:55
0:09:55