filmov
tv
Kinetics: Initial Rates and Integrated Rate Laws

Показать описание
Who likes math! Oh, you don't? Maybe skip this one on kinetics. Unless you have to answer this stuff for class. Then yeah, watch this.
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Kinetics: Initial Rates and Integrated Rate Laws
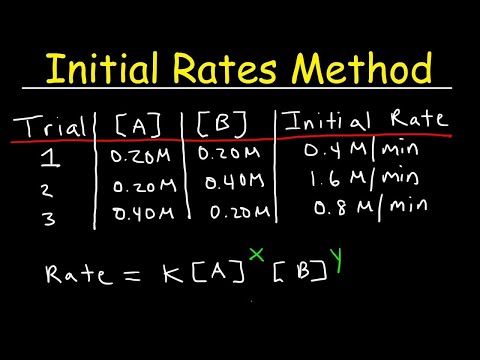
Chemical Kinetics - Initial Rates Method
Integrated Rate Laws - Zero, First, & Second Order Reactions - Chemical Kinetics
Solving a Rate Law Using the Initial Rates Method
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step - Chemical Kinetics
How to Find the Rate Law and Rate Constant (k)
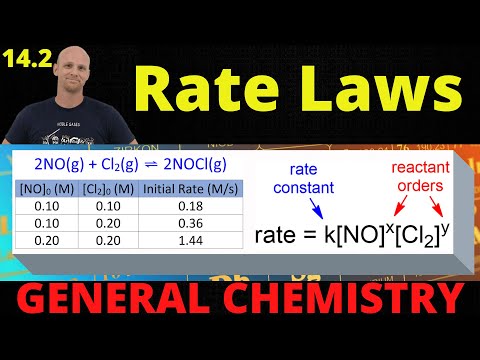
14.2 Rate Laws | General Chemistry
Practice Problem: Initial Rates and Rate Laws
Chemical Engineering | CRE | Lecture - 15 Multiple Reactor System
13.2 The Rate Law
Worked example: Determining a rate law using initial rates data | AP Chemistry | Khan Academy
Reaction Order Tricks & How to Quickly Find the Rate Law
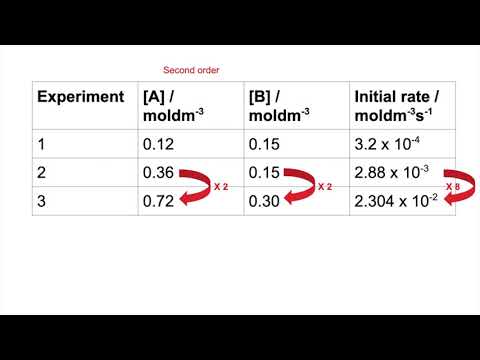
Working out order from a rate table - tricky example
First-order reactions | Kinetics | AP Chemistry | Khan Academy
Kinetics and Reaction Rates (AP Chemistry)
14.5 Integrated Rate Laws | General Chemistry
Chemical Kinetics: Method of Initial Rates First Example
General Chemistry - Integrated Rate Laws - Extra Practice (1 of 2)
Kinetics Rate Constants and Rate Law made super simple (0 order, 1st order, 2nd order) MCAT
Chemical Kinetics: Method of Initial Rates Example
14.5b Derivation of the Integrated Rate Laws | General Chemistry
Integrated Rate Laws - 1st Order and 2nd Order Examples
Finding the Rate Law using Method of Initial Rates Experiments + Example (Part 3)
Using Excel to Determine Chemical Reaction Orders - Chemical Kinetics - First Order Reaction
Комментарии
 0:09:10
0:09:10
 0:34:53
0:34:53
 0:48:46
0:48:46
 0:10:49
0:10:49
 0:18:48
0:18:48
 0:03:42
0:03:42
 0:25:16
0:25:16
 0:09:23
0:09:23
 1:38:56
1:38:56
 0:14:31
0:14:31
 0:12:28
0:12:28
 0:01:58
0:01:58
 0:02:46
0:02:46
 0:07:44
0:07:44
 0:07:18
0:07:18
 0:25:10
0:25:10
 0:09:00
0:09:00
 0:08:47
0:08:47
 0:11:56
0:11:56
 0:12:23
0:12:23
 0:07:34
0:07:34
 0:07:18
0:07:18
 0:14:12
0:14:12
 0:17:57
0:17:57