filmov
tv
R2.2.9 Reaction mechanisms (HL)

Показать описание
This video covers how to deduce to the overall equation from the reaction mechanism and also how to deduce the rate expression from the elementary steps.
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step - Chemical Kinetics
16.1/R2.2.9 Solve problems involving the rate expression [HL IB Chemistry]
16.1/R2.2.6 Reaction mechanism, order of reaction and rate-determining step [HL IB Chemistry]
IB Chemistry Topic 6 Kinetics 16.1 Rate expression and reaction mechanism
Chemical Kinetics and Reaction Mechanism
Reaction Mechanisms (IB Chemistry R2.2)
Kinetics: Rate Laws, Reaction Mechanisms, & Methods of Initial Rates
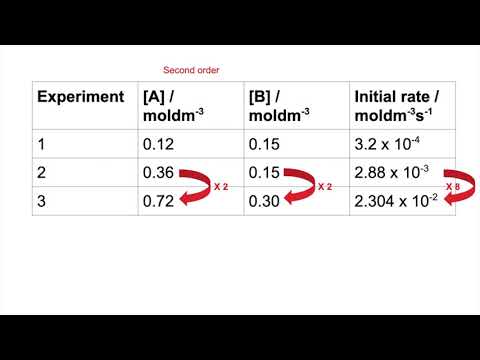
Working out order from a rate table - tricky example
Using Reaction Mechanisms (Rate Limiting Step) to Predict Rate Laws | Chemical Kinetics
What is a rate determining step (RDS)?
Reaction Mechanisms and the Rate Determining step (Quad 2 video)
A level Chemistry Topic by Topic | Rate of a Reaction; Rate order and rate determining step
Reaction Mechanisms: Rate law practice and catalysts
16.1 Catalysts (HL)
Kinetics Part VI (English)
L27D Rate Determining Steps
R2.2.6 Intermediates and catalysts (HL)
Find rate law or order for ANY reaction mechanism: Two important points | Chemical Kinetics
Removing Intermediates from Rate Laws
Molecularity of elementary reaction , rate determining step , reaction intermediate .
Rates and orders 2 - graphical methods and rate-determining step
Reaction Mechanism
#14 Reaction Mechanisms and Rate Law
KAC25.9 - Rates II: Rate Constants from Half-lives
Комментарии
 0:18:48
0:18:48
 0:04:16
0:04:16
 0:09:02
0:09:02
 0:14:03
0:14:03
 0:14:23
0:14:23
 0:18:11
0:18:11
 0:08:45
0:08:45
 0:02:46
0:02:46
 0:29:16
0:29:16
 0:02:43
0:02:43
 0:44:00
0:44:00
 0:44:27
0:44:27
 0:09:29
0:09:29
 0:03:18
0:03:18
 0:18:02
0:18:02
 0:09:41
0:09:41
 0:05:27
0:05:27
 0:09:58
0:09:58
 0:09:28
0:09:28
 0:06:17
0:06:17
 0:08:17
0:08:17
 0:09:11
0:09:11
 0:08:25
0:08:25
 0:03:44
0:03:44