filmov
tv
Calculating Vapor Pressure and Boiling Point

Показать описание
The Clausius-Clapeyron equation can be used to estimate how the boiling point changes with pressure, or how the vapor pressure changes with temperature.
Vapor Pressure - Normal Boiling Point & Clausius Clapeyron Equation
Calculating Vapor Pressure and Boiling Point
Vapor Pressure and Boiling
ALEKS - Calculating Vapor Pressure from Boiling Point and Enthalpy of Vaporization
ALEKS: Calculating vapor pressure from boiling point and enthalpy of vaporization
Raoult's Law - How To Calculate The Vapor Pressure of a Solution
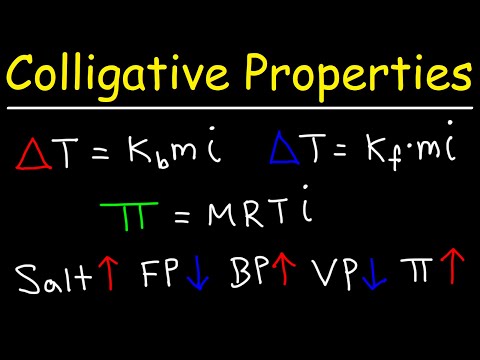
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
2.3 Vapor Pressure, IMFs, and Boiling Point
Molality and Colligative Properties
Simplest Way To Understand Boiling Point & Vapor Pressure
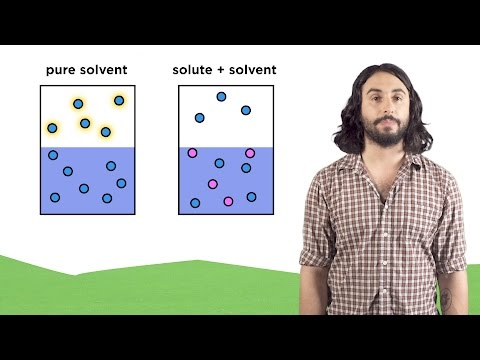
Partial Pressures & Vapor Pressure: Crash Course Chemistry #15
Vapor Pressure
vapor pressure explained
Boiling, Atmospheric Pressure, and Vapor Pressure
How to use a Vapor Pressure Graph for Boiling Point Determinations
Intermolecular Forces and Boiling Points
How To Calculate Vapor Pressure From Boiling Point? - CreditGuide360.com
Vapor Pressure and Boiling Point
Vapor Pressure & Boiling Point
2.4 Reference Table H (Vapor Pressure and Temperature)
Boiling Point Elevation & Vapor Pressure Calculations
Calculate Boiling Point of Titanium at Different Pressure
Evaporation, Vapor Pressure and Boiling
ALEKS: Understanding the connection between vapor pressure, boiling point, enthalpy of vaporization
Комментарии
 0:11:11
0:11:11
 0:12:40
0:12:40
 0:01:54
0:01:54
 0:04:45
0:04:45
 0:06:40
0:06:40
 0:14:02
0:14:02
 0:25:23
0:25:23
 0:06:34
0:06:34
 0:05:10
0:05:10
 0:03:26
0:03:26
 0:11:55
0:11:55
 0:02:17
0:02:17
 0:02:05
0:02:05
 0:06:05
0:06:05
 0:07:55
0:07:55
 0:10:54
0:10:54
 0:02:00
0:02:00
 0:04:25
0:04:25
 0:11:26
0:11:26
 0:06:35
0:06:35
 0:08:16
0:08:16
 0:06:45
0:06:45
 0:11:47
0:11:47
 0:06:02
0:06:02