filmov
tv
ALEKS - Calculating Vapor Pressure from Boiling Point and Enthalpy of Vaporization

Показать описание
ALEKS: Calculating vapor pressure from boiling point and enthalpy of vaporization
ALEKS: Calculating enthalpy of vaporization from vapor pressure
ALEKS - Calculating Vapor Pressure from Boiling Point and Enthalpy of Vaporization
ALEKS - Calculating Enthalpy of Vaporization from Vapor Pressure
ALEKS: Using Raoult’s Law to calculate the vapor pressure of a component
ALEKS: Relating vapor pressure to vaporization
ALEKS: Understanding the connection between vapor pressure, boiling point, enthalpy of vaporization
ALEKS - Relating Vapor Pressure to Vaporization
Aleks Relating vapor pressure to vaporization
ALEKS: Interconverting atmospheres and kilopascal
Vapor Pressure and Boiling
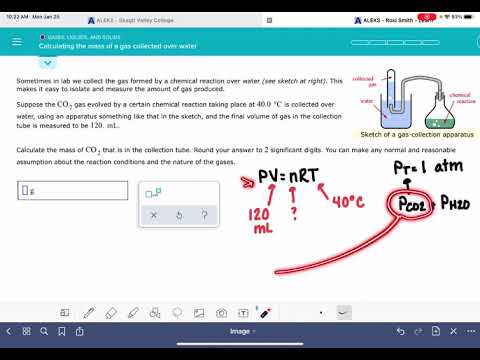
ALEKS: Calculating the mass of a gas collected over water
ALEKS - Calculating ideal solution composition after a distillation
ALEKS: Using Henry's Law to calculate the solubility of a gas
ALEKS: Interconverting atmospheres and torr
ALEKS - Understanding the Connection Between Vapor Pressure, Boiling Point, etc.
15 6d Using Raoult's Law to calculate the vapor pressure of a component
ALEKS: Using heat of fusion or vaporization to find the heat needed to melt or boil a substance
Aleks Calculating partial pressure in a gas mixture
ALEKS: Finding an atomic radius from an fcc or bcc lattice constant
ALEKS: Calculating key distances in the bcc unit cell
ALEKS: Using the Kf and Kb equations with electrolytes
ALEKS - Calculating Solubility
ALEKS: Sketching a described thermodynamic change on a phase diagram
Комментарии
 0:06:40
0:06:40
 0:10:01
0:10:01
 0:04:45
0:04:45
 0:04:07
0:04:07
 0:03:37
0:03:37
 0:04:16
0:04:16
 0:06:02
0:06:02
 0:06:02
0:06:02
 0:11:21
0:11:21
 0:02:45
0:02:45
 0:01:54
0:01:54
 0:07:13
0:07:13
 0:20:38
0:20:38
 0:04:27
0:04:27
 0:02:49
0:02:49
 0:05:30
0:05:30
 0:04:00
0:04:00
 0:11:38
0:11:38
 0:08:31
0:08:31
 0:01:36
0:01:36
 0:07:13
0:07:13
 0:04:42
0:04:42
 0:06:24
0:06:24
 0:04:12
0:04:12