filmov
tv
vapor pressure explained

Показать описание
vapor pressure explained.
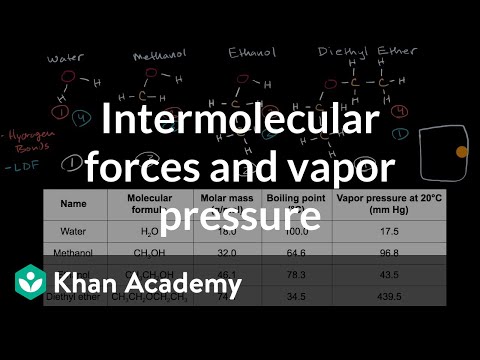
understanding vapor pressure. This video explains vapor pressure and boiling. Vapor pressure or vapour pressure or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of particles to escape from the liquid (or a solid). A substance with a high vapor pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapor also increases, thereby increasing the vapor pressure.
The vapor pressure of any substance increases non-linearly with temperature according to the Clausius–Clapeyron relation. The atmospheric pressure boiling point of a liquid (also known as the normal boiling point) is the temperature at which the vapor pressure equals the ambient atmospheric pressure. With any incremental increase in that temperature, the vapor pressure becomes sufficient to overcome atmospheric pressure and lift the liquid to form vapor bubbles inside the bulk of the substance. Bubble formation deeper in the liquid requires a higher temperature due to the higher fluid pressure, because fluid pressure increases above the atmospheric pressure as the depth increases. More important at shallow depths is the higher temperature required to start bubble formation. The surface tension of the bubble walls leads to an overpressure in the very small initial bubbles.
what is vapor pressure 0:00 to 2:00
what is vapour pressure 0:00 to 2:00
Vapor Pressure.
Vapour pressure.
Vapor pressure chemistry
Vapor pressure chemistry class 11.
Vapor pressure and boiling point.
Vapor pressure lowering.
Vapor pressure in hindi.
Vapor pressure equilibrium.
Vapor pressure and boiling point chemistry.
Vapor pressure animation.
Vapor pressure of liquids.
Vapor pressure and temperature relationship.
Vapor pressure calculation.
Vapor pressure organic chemistry tutor.
Vapor pressure demonstration.
Vapor pressure and cavitation.
#vapor pressure
understanding vapor pressure. This video explains vapor pressure and boiling. Vapor pressure or vapour pressure or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of particles to escape from the liquid (or a solid). A substance with a high vapor pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapor also increases, thereby increasing the vapor pressure.
The vapor pressure of any substance increases non-linearly with temperature according to the Clausius–Clapeyron relation. The atmospheric pressure boiling point of a liquid (also known as the normal boiling point) is the temperature at which the vapor pressure equals the ambient atmospheric pressure. With any incremental increase in that temperature, the vapor pressure becomes sufficient to overcome atmospheric pressure and lift the liquid to form vapor bubbles inside the bulk of the substance. Bubble formation deeper in the liquid requires a higher temperature due to the higher fluid pressure, because fluid pressure increases above the atmospheric pressure as the depth increases. More important at shallow depths is the higher temperature required to start bubble formation. The surface tension of the bubble walls leads to an overpressure in the very small initial bubbles.
what is vapor pressure 0:00 to 2:00
what is vapour pressure 0:00 to 2:00
Vapor Pressure.
Vapour pressure.
Vapor pressure chemistry
Vapor pressure chemistry class 11.
Vapor pressure and boiling point.
Vapor pressure lowering.
Vapor pressure in hindi.
Vapor pressure equilibrium.
Vapor pressure and boiling point chemistry.
Vapor pressure animation.
Vapor pressure of liquids.
Vapor pressure and temperature relationship.
Vapor pressure calculation.
Vapor pressure organic chemistry tutor.
Vapor pressure demonstration.
Vapor pressure and cavitation.
#vapor pressure
Комментарии
 0:02:05
0:02:05
 0:01:54
0:01:54
 0:02:17
0:02:17
 0:11:55
0:11:55
 0:03:49
0:03:49
 0:11:11
0:11:11
 0:06:05
0:06:05
 0:04:50
0:04:50
 0:06:34
0:06:34
 0:03:26
0:03:26
 0:06:04
0:06:04
 0:03:21
0:03:21
 0:04:18
0:04:18
 0:05:38
0:05:38
 0:00:59
0:00:59
 0:17:21
0:17:21
 0:14:02
0:14:02
 0:06:02
0:06:02
 0:00:58
0:00:58
 0:02:14
0:02:14
 0:00:55
0:00:55
 0:00:32
0:00:32
 0:09:29
0:09:29
 0:02:17
0:02:17