filmov
tv
Vapor Pressure and Boiling

Показать описание
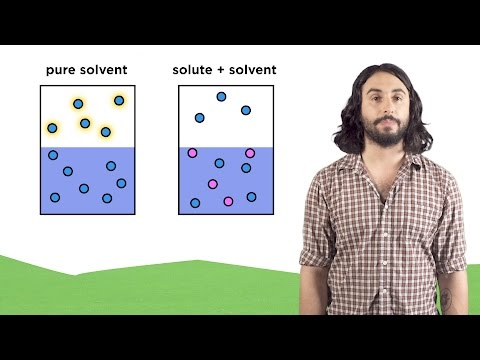
The molecules leaving a liquid through evaporation create an upward pressure as they collide with air molecules. This upward push is called the vapor pressure.
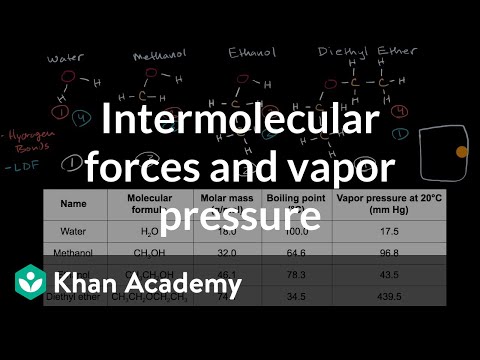
Different substances have different vapor pressures and therefore different boiling points. This is due to differing intermolecular forces between molecules.
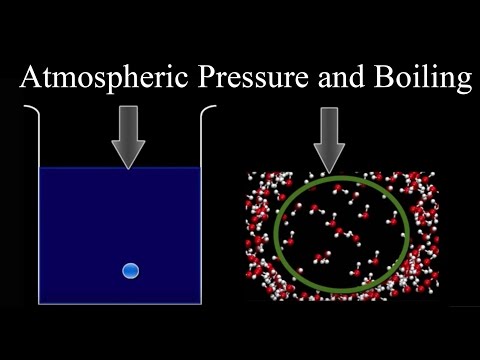
The vapor pressure of a liquid lowers the amount of pressure exerted on the liquid by the atmosphere. As a result, liquids with high vapor pressures have lower boiling points.
Vapor pressure can be increased by heating a liquid and causing more molecules to enter the atmosphere. At the point where the vapor pressure is equal to the atmospheric pressure boiling will begin. In effect, without any external pressure the liquid molecules will be able to spread out and change from a liquid to a gaseous phase. The gas, as bubbles in the liquid, will rise to the surface and be released into the atmosphere.
Different substances have different vapor pressures and therefore different boiling points. This is due to differing intermolecular forces between molecules.
The vapor pressure of a liquid lowers the amount of pressure exerted on the liquid by the atmosphere. As a result, liquids with high vapor pressures have lower boiling points.
Vapor pressure can be increased by heating a liquid and causing more molecules to enter the atmosphere. At the point where the vapor pressure is equal to the atmospheric pressure boiling will begin. In effect, without any external pressure the liquid molecules will be able to spread out and change from a liquid to a gaseous phase. The gas, as bubbles in the liquid, will rise to the surface and be released into the atmosphere.
Vapor Pressure and Boiling
Simplest Way To Understand Boiling Point & Vapor Pressure
Boiling, Atmospheric Pressure, and Vapor Pressure
Evaporation, Vapor Pressure and Boiling
2.3 Vapor Pressure, IMFs, and Boiling Point
Vapor Pressure - Normal Boiling Point & Clausius Clapeyron Equation
Calculating Vapor Pressure and Boiling Point
Vapor Pressure & Boiling Point
Vapor Pressure and Boiling
Vapor Pressure and Boiling Point
29: Vapor pressure and boiling point
ALEKS - Calculating Vapor Pressure from Boiling Point and Enthalpy of Vaporization
Vapor Pressure & Boiling Point
Molality and Colligative Properties
Partial Pressures & Vapor Pressure: Crash Course Chemistry #15
Vapor Pressure
vapor pressure explained
Boiling Water Without Heat | Earth Science
Intermolecular forces and vapor pressure | Intermolecular forces | AP Chemistry | Khan Academy
Atmospheric Pressure and Boiling
What is the relationship between boiling point and vapor pressure?
Calculate Vapor Pressure and Boiling Point using Aspen HYSYS - Lecture # 11
VAPOR PRESSURE and BOILING
Boiling Point and Vapor Pressure: Chapter 11 – Part 5
Комментарии
 0:01:54
0:01:54
 0:03:26
0:03:26
 0:06:05
0:06:05
 0:11:47
0:11:47
 0:06:34
0:06:34
 0:11:11
0:11:11
 0:12:40
0:12:40
 0:11:26
0:11:26
 0:03:14
0:03:14
 0:04:25
0:04:25
 0:07:29
0:07:29
 0:04:45
0:04:45
 0:07:58
0:07:58
 0:05:10
0:05:10
 0:11:55
0:11:55
 0:02:17
0:02:17
 0:02:05
0:02:05
 0:02:58
0:02:58
 0:06:04
0:06:04
 0:02:53
0:02:53
 0:05:09
0:05:09
 0:04:01
0:04:01
 0:03:33
0:03:33
 0:04:57
0:04:57