filmov
tv
ALEKS: Understanding the connection between vapor pressure, boiling point, enthalpy of vaporization

Показать описание
Substances with high vapor pressure have low boiling points and low enthalpies of vaporization.
ALEKS - Understanding the Connection Between Vapor Pressure, Boiling Point, etc.
ALEKS: Understanding the connection between vapor pressure, boiling point, enthalpy of vaporization
Aleks Understanding connections between descriptions of weak acid dissociation
ALEKS: Understanding the relationship between DNA and mRNA base sequences
ALEKS: Understanding the definitions of heat and work
ALEKS: Understanding the definition of enthalpy
ALEKS: Understanding the relationship between mass, volume, and density
ALEKS: Understanding the origin of the van der Waals equation of state
Aleks Understanding the effect of induction on acidity
ALEKS: Understanding the definitions of ionization energy and electron affinity
Aleks Understanding conceptual components of the enthalpy of solution
ALEKS: Understanding how solubility varies with temperature and pressure
ALEKS: Understanding how electrostatic forces cancel
ALEKS: Understanding the common modes of radioactive decay
ALEKS: Understanding how molecular collision rate scales with temperature and volume
ALEKS: Understanding the qualitative predictions of the Arrhenius equation
ALEKS: Understanding the qualitative predictions of the Arrhenius equation
ALEKS: Understanding how average molecular speed scales with temperature and molar mass
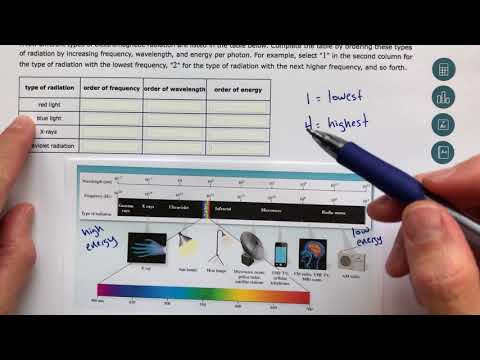
ALEKS - Understanding the Organization of the Electromagnetic Spectrum
ALEKS: Understanding the structure of nucleic acid strands
ALEKS: Understanding net electrical charge
ALEKS: Understanding theoretical, actual, and percent yield
ALEKS: Understanding consequences of important physical properties of liquids
ALEKS: Understanding the meaning of a de Broglie wavelength
Комментарии
 0:05:30
0:05:30
 0:06:02
0:06:02
 0:04:01
0:04:01
 0:01:39
0:01:39
 0:05:55
0:05:55
 0:03:07
0:03:07
 0:07:20
0:07:20
 0:04:12
0:04:12
 0:06:19
0:06:19
 0:05:15
0:05:15
 0:12:28
0:12:28
 0:05:40
0:05:40
 0:07:19
0:07:19
 0:05:38
0:05:38
 0:03:50
0:03:50
 0:05:33
0:05:33
 0:03:15
0:03:15
 0:03:00
0:03:00
 0:05:28
0:05:28
 0:02:54
0:02:54
 0:03:57
0:03:57
 0:06:58
0:06:58
 0:07:31
0:07:31
 0:07:35
0:07:35