filmov
tv
Atomic Orbitals Simply Explained! Inorganic CHEM - 1.12

Показать описание
The course I'm teaching is a distilled version of the material covered in the following textbooks, which I highly reccommend:
This video simply explains atomic orbitals. Please note that the information in this video may not always be accurate or up-to-date due to new discoveries. There may also be overlooked mistakes. Therefore, only use this video as an additional resource to enhance your understanding.
This video simply explains atomic orbitals. Please note that the information in this video may not always be accurate or up-to-date due to new discoveries. There may also be overlooked mistakes. Therefore, only use this video as an additional resource to enhance your understanding.
Atomic Orbitals Simply Explained! Inorganic CHEM - 1.12
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Orbitals: Crash Course Chemistry #25
Shells, Subshells, and Orbitals - BIOLOGY/CHEMISTRY EP5
Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3
The Shapes of Atomic Orbitals s-orbital, p-orbital and d-orbital
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
Electron Configuration - Basic introduction
What are Shells, Subshells, and Orbitals? | Chemistry
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
Molecular Orbital Theory - Bonding & Antibonding MO - Bond Order
Chemistry - Atomic Structure - EXPLAINED!
The Schrödinger Equation Explained in 60 Seconds
Valence Bond Theory & Hybrid Atomic Orbitals
VSEPR Theory - Basic Introduction
Photon momentum, wave-particle duality, & Bohr atom simply explained! Inorganic CHEM - 1.8
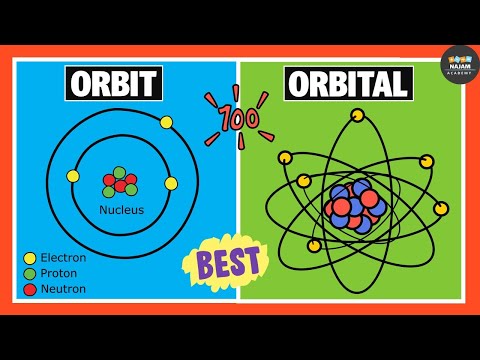
Difference Between Orbits and Orbitals | Chemistry
Orbital Diagrams and Electron Configuration - Basic Introduction - Chemistry Practice Problems
Hybridization of Atomic Orbitals | SP, SP2, SP3 Hybridization of Carbon
12. Molecular Orbitals (Intro to Solid-State Chemistry)
How small are atoms?
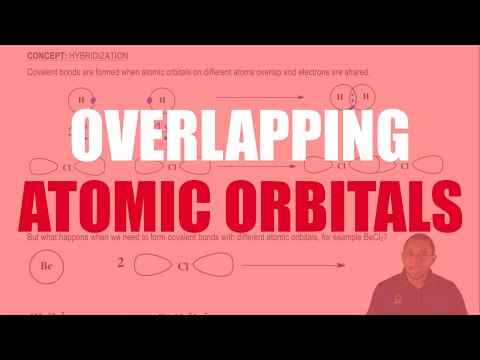
Understanding Overlapping Atomic Orbitals
Atomic Orbitals Practice Questions! Inorganic CHEM - 1.13
Комментарии
 0:05:56
0:05:56
 0:11:19
0:11:19
 0:08:42
0:08:42
 0:10:52
0:10:52
 0:09:23
0:09:23
 0:10:55
0:10:55
 0:10:53
0:10:53
 0:07:54
0:07:54
 0:10:19
0:10:19
 0:06:00
0:06:00
 0:07:53
0:07:53
 0:21:36
0:21:36
 0:11:45
0:11:45
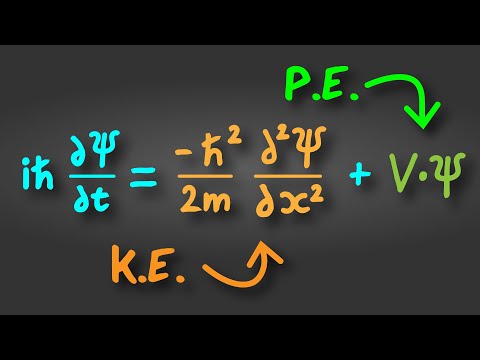 0:01:00
0:01:00
 0:10:39
0:10:39
 0:13:10
0:13:10
 0:20:08
0:20:08
 0:08:11
0:08:11
 0:12:12
0:12:12
 0:13:48
0:13:48
 0:48:53
0:48:53
 0:00:48
0:00:48
 0:02:21
0:02:21
 0:06:58
0:06:58