filmov
tv
The Schrödinger Equation Explained in 60 Seconds

Показать описание
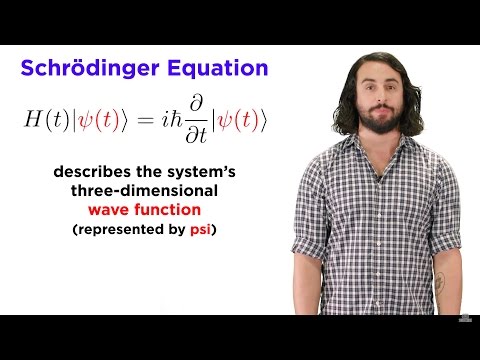
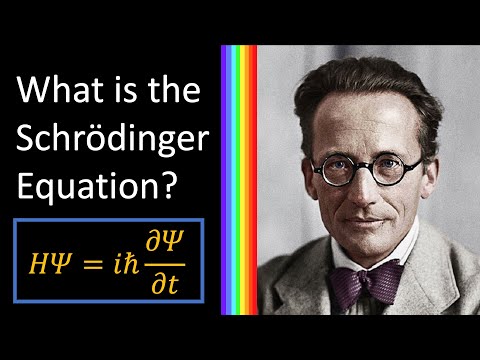
The Schrödinger Equation is the key equation in quantum physics that explains how particles in quantum physics behave. You wanna know where your electron is going to go? That’s the Schrödinger equation you’ll need.
So why does it look so complicated? Well it’s because in quantum physics, when you are describing what a thing like an electron will do, it doesn’t follow the rules of particles it follows the rules of waves, and that’s what these psi’s are, they are wave functions.
Okay so an electron is a wave, and a wave can evolve in space and in time, but it can’t evolve in any old way, there are constraints about how it can evolve, and those constraints? That’s what the Schrödinger equation is telling us.
It says that over time the total energy has to stay the same. And this total energy is made of the kinetic energy, and potential energy. An example of a potential energy for an electron is just being in an atom.
So if you want to see what a quantum object will do in the future, you need to find the wave function that will satisfy all these bits of the Schrödinger equation.
#quantum #physics #DomainOfScience
--- Posters ----
-- Some Awesome People ---
And many thanks to my $10 supporters on Patreon, you are awesome!
Theodore Chu
Petr Murmak
Sebastian
Eric Epstein
Alex Polo
Kevin Delaney
Reggie Fourmyle
Mark Pickenheim
Raj Duphare
Terrence Masson
Join the gang and help support me produce free and high quality science content:
--- My Science Books ----
I also write science books for kids called Professor Astro Cat. You can see them all here:
--- Follow me around the internet ---
--- Credits ---
Music, art, and everything else by Dominic Walliman
So why does it look so complicated? Well it’s because in quantum physics, when you are describing what a thing like an electron will do, it doesn’t follow the rules of particles it follows the rules of waves, and that’s what these psi’s are, they are wave functions.
Okay so an electron is a wave, and a wave can evolve in space and in time, but it can’t evolve in any old way, there are constraints about how it can evolve, and those constraints? That’s what the Schrödinger equation is telling us.
It says that over time the total energy has to stay the same. And this total energy is made of the kinetic energy, and potential energy. An example of a potential energy for an electron is just being in an atom.
So if you want to see what a quantum object will do in the future, you need to find the wave function that will satisfy all these bits of the Schrödinger equation.
#quantum #physics #DomainOfScience
--- Posters ----
-- Some Awesome People ---
And many thanks to my $10 supporters on Patreon, you are awesome!
Theodore Chu
Petr Murmak
Sebastian
Eric Epstein
Alex Polo
Kevin Delaney
Reggie Fourmyle
Mark Pickenheim
Raj Duphare
Terrence Masson
Join the gang and help support me produce free and high quality science content:
--- My Science Books ----
I also write science books for kids called Professor Astro Cat. You can see them all here:
--- Follow me around the internet ---
--- Credits ---
Music, art, and everything else by Dominic Walliman
Комментарии
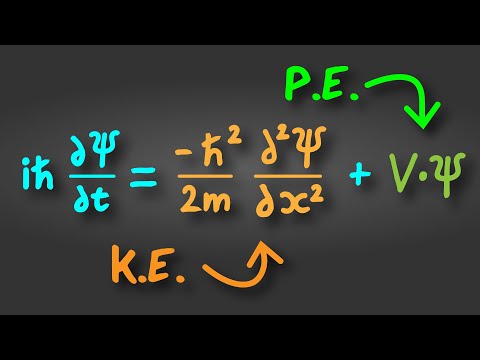 0:01:00
0:01:00
 0:06:28
0:06:28
 0:14:13
0:14:13
 0:09:28
0:09:28
 0:08:45
0:08:45
 1:27:34
1:27:34
 0:12:19
0:12:19
 0:49:30
0:49:30
 1:25:54
1:25:54
 0:16:35
0:16:35
 0:13:04
0:13:04
 0:14:58
0:14:58
 0:10:11
0:10:11
 0:29:55
0:29:55
 0:08:58
0:08:58
 1:22:19
1:22:19
 0:00:58
0:00:58
 0:55:36
0:55:36
 0:18:40
0:18:40
 0:20:59
0:20:59
 0:01:22
0:01:22
 0:06:34
0:06:34
 0:12:57
0:12:57
 0:14:08
0:14:08