filmov
tv
The Shapes of Atomic Orbitals s-orbital, p-orbital and d-orbital

Показать описание
This lecture is about the shapes of atomic orbitals. In this animated tutorial, I will teach you the easy concept of shape of s orbital, shape of p orbital, shape of d orbital, etc.
Q: What is the shape of s orbital?
Ans: The shape of s orbital is like sphere. S orbital can accommodate only two electrons. Remember that the size of p-orbital is bigger than s-orbital and that of d-orbital is bigger than p-orbital.
Q: What is the shape of p orbital?
Ans: The shape of p orbital is like dumbbell. P orbital can accommodate six electrons. P orbital has three degenerate orbitals like, 2px, 2py and 2pz. Each orbital can hold only two electrons.
Q: What is the shape of d orbital?
Ans: The shape of d orbital is like double dumbbell. D orbital can accommodate 10 electrons. D-orbital has five degenerate orbitals.
#shapesoforbitals
#atomicorbitals
#chemistry
To learn more about the shapes of atomic orbitals, watch this lecture till the end.
Q: What is the shape of s orbital?
Ans: The shape of s orbital is like sphere. S orbital can accommodate only two electrons. Remember that the size of p-orbital is bigger than s-orbital and that of d-orbital is bigger than p-orbital.
Q: What is the shape of p orbital?
Ans: The shape of p orbital is like dumbbell. P orbital can accommodate six electrons. P orbital has three degenerate orbitals like, 2px, 2py and 2pz. Each orbital can hold only two electrons.
Q: What is the shape of d orbital?
Ans: The shape of d orbital is like double dumbbell. D orbital can accommodate 10 electrons. D-orbital has five degenerate orbitals.
#shapesoforbitals
#atomicorbitals
#chemistry
To learn more about the shapes of atomic orbitals, watch this lecture till the end.
Комментарии
 0:10:53
0:10:53
 0:07:37
0:07:37
 0:14:28
0:14:28
 0:11:19
0:11:19
 0:21:34
0:21:34
 0:05:35
0:05:35
 0:06:05
0:06:05
 0:02:50
0:02:50
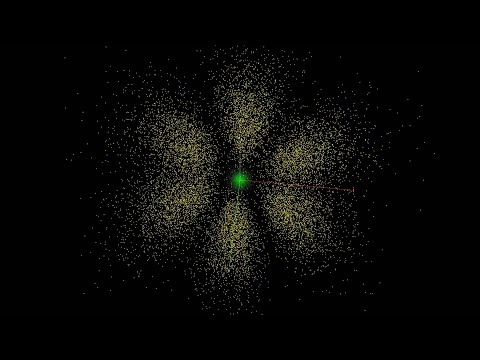 0:05:50
0:05:50
 0:08:42
0:08:42
 0:08:39
0:08:39
 0:10:52
0:10:52
 0:09:44
0:09:44
 0:09:34
0:09:34
 0:05:56
0:05:56
 0:07:44
0:07:44
 0:00:33
0:00:33
 0:05:42
0:05:42
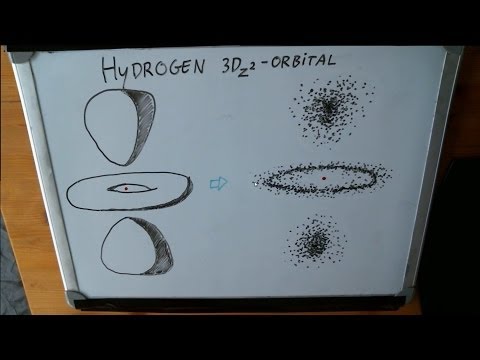 0:07:18
0:07:18
 0:02:25
0:02:25
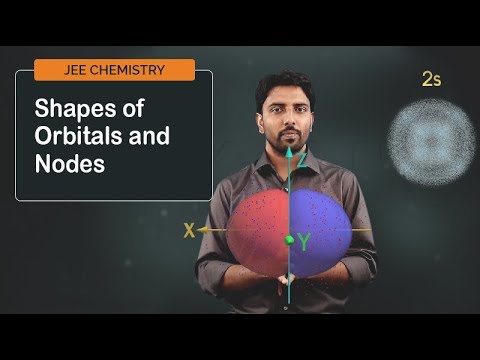 0:02:50
0:02:50
 0:06:00
0:06:00
 0:12:42
0:12:42
 0:00:16
0:00:16