filmov
tv
Difference Between Orbits and Orbitals | Chemistry

Показать описание
In this animated lecture, I will teach you about difference between orbit and orbital in chemistry. Also, you will learn that how there are different orbits around the nucleus and their respective orbitals.
Q: What is an Orbit?
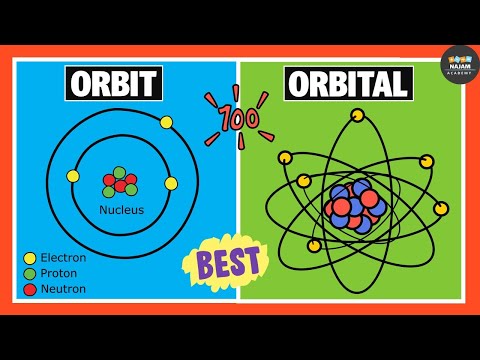
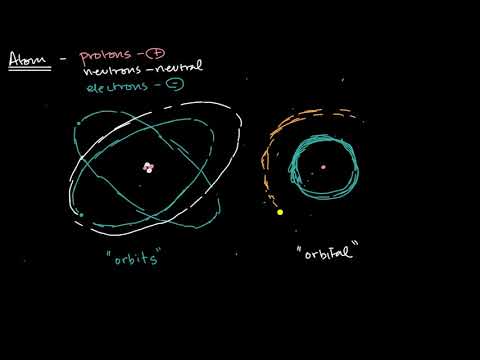
Ans: The fixed circular paths around the nucleus where electrons revolve are called orbits. Just like planets, electrons also revolve around the nucleus over fixed circular paths. These fixed circular paths are known as orbits. Orbits are represented by "n".
For example,
when n=1 it is K-orbit
when n=2 it is L-orbit
when n=3 it is M-orbit
when n=4 it is N-orbit
Q: What is an orbital?
Ans: The 3 dimensional region around the nucleus where probability of finding an electron is maximum is called orbital. Orbitals are the spherical or dumble like region around the nucleus where we can locate the position of an electron. Orbitals are represented by s, p, d, f.
For example, 1st or K-orbita has only one orbital "1s". 2nd or L-orbit has 2 orbitals "2s,2p", etc.
Q: What is the difference between orbit and orbital?
Ans: Remember that orbits are you the fixed circular paths around the nucleus where electrons revolve. Orbitals are the 3 dimensional region around thr nucleus where probability of finding an electron is maximum.
To learn more about orbit and orbital, watch this lecture till the end.
#DifferenceBetweenOrbitAndOrbital
#Orbit
#Orbital
Q: What is an Orbit?
Ans: The fixed circular paths around the nucleus where electrons revolve are called orbits. Just like planets, electrons also revolve around the nucleus over fixed circular paths. These fixed circular paths are known as orbits. Orbits are represented by "n".
For example,
when n=1 it is K-orbit
when n=2 it is L-orbit
when n=3 it is M-orbit
when n=4 it is N-orbit
Q: What is an orbital?
Ans: The 3 dimensional region around the nucleus where probability of finding an electron is maximum is called orbital. Orbitals are the spherical or dumble like region around the nucleus where we can locate the position of an electron. Orbitals are represented by s, p, d, f.
For example, 1st or K-orbita has only one orbital "1s". 2nd or L-orbit has 2 orbitals "2s,2p", etc.
Q: What is the difference between orbit and orbital?
Ans: Remember that orbits are you the fixed circular paths around the nucleus where electrons revolve. Orbitals are the 3 dimensional region around thr nucleus where probability of finding an electron is maximum.
To learn more about orbit and orbital, watch this lecture till the end.
#DifferenceBetweenOrbitAndOrbital
#Orbit
#Orbital
Комментарии
 0:08:11
0:08:11
 0:02:14
0:02:14
 0:06:00
0:06:00
 0:02:50
0:02:50
 0:09:41
0:09:41
 0:11:19
0:11:19
 0:21:34
0:21:34
 0:02:36
0:02:36
 0:07:38
0:07:38
 0:20:44
0:20:44
 0:05:50
0:05:50
 0:04:06
0:04:06
 0:09:23
0:09:23
 0:09:47
0:09:47
 0:00:48
0:00:48
 0:06:11
0:06:11
 0:00:56
0:00:56
 0:05:35
0:05:35
 0:08:42
0:08:42
 0:00:52
0:00:52
 0:08:39
0:08:39
 0:04:52
0:04:52
 0:04:58
0:04:58
 0:03:19
0:03:19