filmov
tv
Calculating the Resulting pH

Показать описание
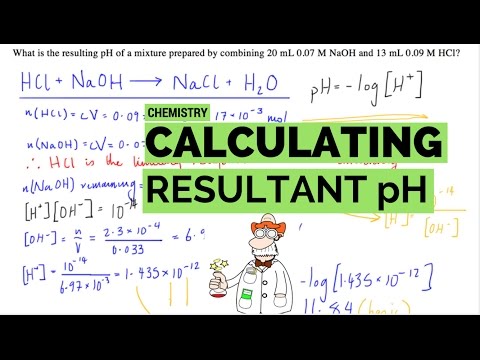
Calculating the pH of the resulting solution when Hydrochloric Acid is mixed with Sodium Hydroxide. First step is to calculate which solute is in excess and how much of the excess reagent is remaining after neutralisation has occurred.
From this we then were able to use the equilibrium constant for water to determine the concentration of hydrogen ions were remaining in solution and then from that calculate the resultant pH of the solution.
I hope the example helps :)
From this we then were able to use the equilibrium constant for water to determine the concentration of hydrogen ions were remaining in solution and then from that calculate the resultant pH of the solution.
I hope the example helps :)
Calculating the Resulting pH
Calculating pH of a Resulting Solution
pH of Weak Acids and Bases - Percent Ionization - Ka & Kb
How To Calculate The pH of a Solution Without a Calculator - Acids and Bases
Buffer solution pH calculations | Chemistry | Khan Academy
Calculating the pH of a Resulting Solution - Neutralisation Reaction
Calculating pH of Resulting Solution
How to Calculate the pH of a Solution
Calculate the \( \mathrm{pH} \) of the resultant mixtures: a) \( 10...
Find the pH of a Buffer after Adding HCl
pH of Two Mixed Solutions
Resultant ph
Calculate resultant pH after neutralisation | Acids and bases | meriSTEM
Calculate the pH of Acids and Bases Given the Concentration of a Solution
pH at Equivalence Point
Worked example: Calculating the pH after a weak acid–strong base reaction (excess acid)
Calculating the pH of a solution made up of 2 acids of different volumes and concentrations
pH Calculations
Find the pH of a Buffer after adding NaOH
Practice Problem: Calculations Involving pH and Ka
Calculating pH After Mixing Acid & Base // HSC Chemistry
Worked examples: Calculating [H₃O⁺] and pH | Acids and bases | AP Chemistry | Khan Academy
Calculating the pH during a strong acid-strong base titration
Weak Acid / Strong Base Titration - All pH Calculations
Комментарии
 0:12:02
0:12:02
 0:12:13
0:12:13
 0:29:31
0:29:31
 0:21:09
0:21:09
 0:11:39
0:11:39
 0:16:35
0:16:35
 0:07:05
0:07:05
 0:03:32
0:03:32
 0:06:58
0:06:58
 0:04:43
0:04:43
 0:09:25
0:09:25
 0:04:43
0:04:43
 0:04:56
0:04:56
 0:28:17
0:28:17
 0:07:14
0:07:14
 0:09:04
0:09:04
 0:03:32
0:03:32
 0:04:14
0:04:14
 0:05:25
0:05:25
 0:06:03
0:06:03
 0:14:38
0:14:38
 0:07:05
0:07:05
 0:08:52
0:08:52
 0:18:52
0:18:52