filmov
tv
Calculating the pH of a solution made up of 2 acids of different volumes and concentrations

Показать описание
By the end of this video, you should be able to calcule the pH of a solution made up of 2 acids of different volumes and concentrations.
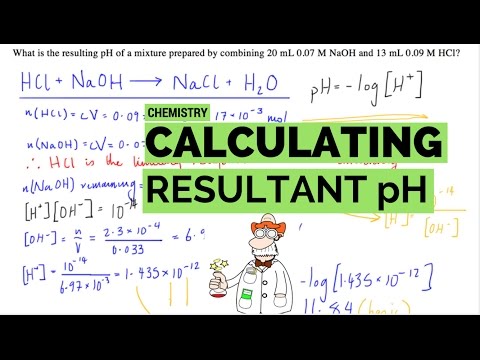
Calculate the pH of the resulting solution when 300 cm3 of a solution containing a hydrogen ion concentration of 0.200 mol dm-3 is completely mixed with 150 cm3 of a solution containing a hydrogen ion concentration of 0.100 mol dm-3
ANS
ANS
1. Calculate the number of moles of hydrogen ions for each solution through n=cv
n1= C1V1= (0.2)(300 x 10-3) = 0.06 mol n2= C2V2= (0.1)(150 x 10-3) = 0.015 mol
2. Add the number of moles for each solution together.
n = n1 + n2 = 0.06 + 0.015 = 0.075 mol of H+ in 450 cm3
3. Calculate the concentration of hydrogen ions [H+]= 𝐧/𝐕 where v is the total volume of the 2 solutions.
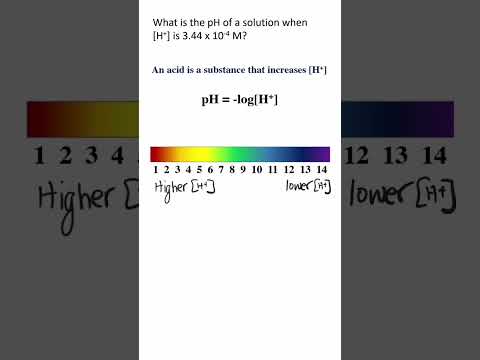
4. pH= -log[H+]
pH = -log[0.167] = 0.78
Calculate the pH of the resulting solution when 300 cm3 of a solution containing a hydrogen ion concentration of 0.200 mol dm-3 is completely mixed with 150 cm3 of a solution containing a hydrogen ion concentration of 0.100 mol dm-3
ANS
ANS
1. Calculate the number of moles of hydrogen ions for each solution through n=cv
n1= C1V1= (0.2)(300 x 10-3) = 0.06 mol n2= C2V2= (0.1)(150 x 10-3) = 0.015 mol
2. Add the number of moles for each solution together.
n = n1 + n2 = 0.06 + 0.015 = 0.075 mol of H+ in 450 cm3
3. Calculate the concentration of hydrogen ions [H+]= 𝐧/𝐕 where v is the total volume of the 2 solutions.
4. pH= -log[H+]
pH = -log[0.167] = 0.78
 0:13:50
0:13:50
 0:08:36
0:08:36
 0:21:09
0:21:09
 0:04:05
0:04:05
 0:11:23
0:11:23
 0:02:54
0:02:54
 0:09:54
0:09:54
 0:29:31
0:29:31
 0:03:14
0:03:14
 0:14:40
0:14:40
 0:11:26
0:11:26
 0:22:56
0:22:56
 0:07:05
0:07:05
 0:07:33
0:07:33
 0:37:42
0:37:42
 0:12:22
0:12:22
 0:00:47
0:00:47
 0:09:01
0:09:01
 0:12:02
0:12:02
 0:00:59
0:00:59
 0:08:51
0:08:51
 0:04:14
0:04:14
 0:12:37
0:12:37
 0:28:17
0:28:17