filmov
tv
Gibbs Free Energy and Calculations

Показать описание
Explore Channels, available in Pearson+, and access thousands of videos with bite-sized lessons in multiple college courses. The videos are hand-picked, and led by experts, to help you learn faster and easier. Understand tricky concepts, quiz yourself with practice questions, download worksheets to follow along with the lessons and stay on top of your studies. Plus, experts are available for Q&A. Whether you want it paired with your eTextbook in Pearson+, or watch videos on your own time to go from "huh?" to "aha!" in class, Channels is available to you anytime you need it. It's reimagined learning, designed for your learning style.
---------------
----------------
SOCIAL:
----------------
#PearsonPlus #ThisIsYouLearning #studyvideo #StudyHelp #studying #studytutorial #eTextbooks #StudyToolsForLess #MoreBooksFewerBucks #BingeWatchGoodGrades
---------------
----------------
SOCIAL:
----------------
#PearsonPlus #ThisIsYouLearning #studyvideo #StudyHelp #studying #studytutorial #eTextbooks #StudyToolsForLess #MoreBooksFewerBucks #BingeWatchGoodGrades
Gibbs Free Energy and Calculations
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K
Introduction to Gibbs free energy | Applications of thermodynamics | AP Chemistry | Khan Academy
Using Gibbs Free Energy
How to Calculate ∆G° Standard Gibb's Free Energy Change of a Reaction from Enthalpy H and Entro...
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Cell Potential & Gibbs Free Energy, Standard Reduction Potentials, Electrochemistry Problems
Practice Problem: Calculating Free Energy Change
Up board class 12chemistry chapter 3 numerical trick ।educator coach ।
18.5 Gibbs Free Energy and the Equilibrium Constant
Gibbs Free Energy, Entropy, and Enthalpy
16. Thermodynamics: Gibbs Free Energy and Entropy
Gibbs Free energy change 1
How to Calculate Change in Gibb's Free Energy of Reaction using Gibbs Formation Example & P...
Gibbs Free Energy
Gibbs Free Energy, Entropy, Thermochemistry Question, Percent Composition, Bohr's Atomic Model
18.5 Gibbs Free Energy and the Equilibrium Constant | General Chemistry
Free energy of formation | Applications of thermodynamics | AP Chemistry | Khan Academy
How can you use Gibbs free energy to calculate a unary phase diagram
17.1 Equilibrium and Gibbs free energy (HL)
Calculating Keq from Gibbs Free Energy
Fundamental Equation (Gibbs Energy)
Thermodynamics: Gibbs Free Energy Calculation - Exam Question|A Level Chemistry (AQA)
Gibbs Free Energy Calculator
Комментарии
 0:06:01
0:06:01
 0:44:45
0:44:45
 0:05:39
0:05:39
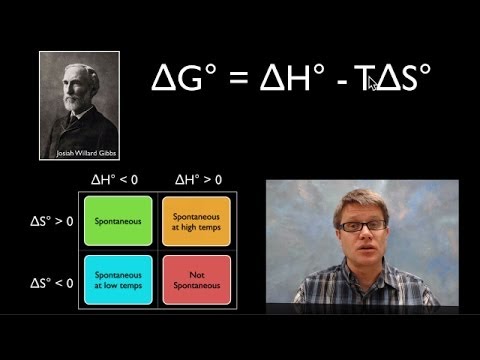 0:07:57
0:07:57
 0:04:05
0:04:05
 0:08:12
0:08:12
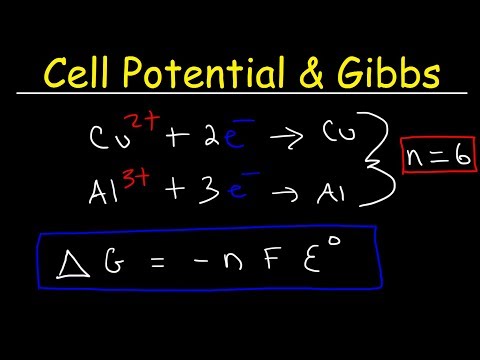 0:11:02
0:11:02
 0:05:12
0:05:12
 0:09:41
0:09:41
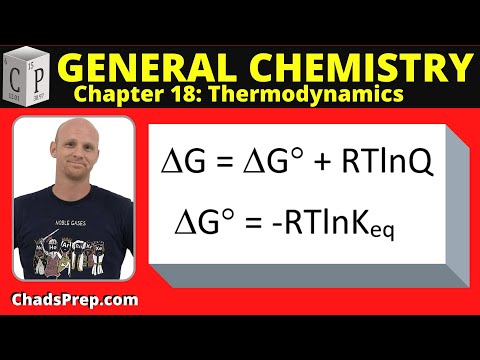 0:11:12
0:11:12
 0:05:33
0:05:33
 0:32:01
0:32:01
 0:11:19
0:11:19
 0:03:29
0:03:29
 0:14:13
0:14:13
 0:48:43
0:48:43
 0:24:43
0:24:43
 0:05:26
0:05:26
 0:08:10
0:08:10
 0:05:21
0:05:21
 0:05:25
0:05:25
 0:04:06
0:04:06
 0:08:56
0:08:56
 0:01:06
0:01:06