filmov
tv
Practice Problem: Calculating Free Energy Change

Показать описание
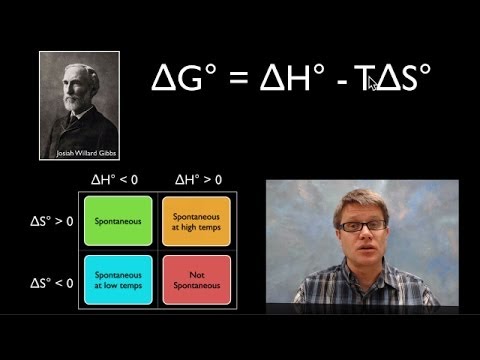
We know that the free energy change associated with a process will tell us whether it is spontaneous or not. And we also know that this depends on the change in enthalpy and the change in entropy for that process, as well as the temperature. Give some thermodynamic data, can we calculate the change in free energy for a process? Let's try!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Practice Problem: Calculating Free Energy Change
Gibbs Free Energy and Calculations
Practice Problem: Cell Potential, Equilibrium Constants, and Free Energy Change
How to Calculate ∆G° Standard Gibb's Free Energy Change of a Reaction from Enthalpy H and Entro...
Introduction to Gibbs free energy | Applications of thermodynamics | AP Chemistry | Khan Academy
Using Gibbs Free Energy
Calculating the Standard Free Energy change for a Stepwise Reaction: Practice Problem #1 (Updated)
Gibbs Free Energy and Calculations: Example 1
Fundamentals of Engineering FE Exam Free Practice Test and Answer
Gibbs Free Energy
Gibbs Free Energy Problems
When you calculate Gibbs free energy and it's not spontaneous 😂
How to Calculate Change in Gibb's Free Energy of Reaction using Gibbs Formation Example & P...
Free energy of formation | Applications of thermodynamics | AP Chemistry | Khan Academy
Gibbs Free Energy and Calculations: Example 3
Gibbs Free Energy and Calculations: Example 6
12.21 sample problem calculate free energy change given Ksp
Gibbs Free Energy and Calculations: Example 5
18.5 Gibbs Free Energy and the Equilibrium Constant | General Chemistry
Gibbs Free Energy, Entropy, Thermochemistry Question, Percent Composition, Bohr's Atomic Model
Calculating Keq from Gibbs Free Energy
Gibbs Free Energy and Calculations: Example 4
Calculating the Standard Change in Free Energy for a Reaction Practice Problem #1 (Updated)
Gibbs Free Energy Equation and Spontaneity in Chemical Reactions (Exam Problems)
Комментарии
 0:05:12
0:05:12
 0:06:01
0:06:01
 0:05:53
0:05:53
 0:04:05
0:04:05
 0:05:39
0:05:39
 0:07:57
0:07:57
 0:11:58
0:11:58
 0:03:55
0:03:55
 1:00:49
1:00:49
 0:14:13
0:14:13
 0:20:26
0:20:26
 0:00:06
0:00:06
 0:03:29
0:03:29
 0:05:26
0:05:26
 0:03:26
0:03:26
 0:04:02
0:04:02
 0:00:56
0:00:56
 0:02:38
0:02:38
 0:24:43
0:24:43
 0:48:43
0:48:43
 0:05:25
0:05:25
 0:04:09
0:04:09
 0:15:45
0:15:45
 0:08:51
0:08:51