filmov
tv
Hybridization and VSEPR Theory

Показать описание
In this video we're looking at the the fascinating world of electron configurations, hybridization, and the VSEPR theory. Beginning with the electron configuration of the carbon atom, we explore the significance of valence electrons, their visualization, and the role they play in chemical bonding. Discover the intricate relationship between the Valence Bond Theory, the shapes of atomic orbitals, and their contributions to molecular structures.
Key Highlights:
Carbon Atom Electron Configuration 🧪
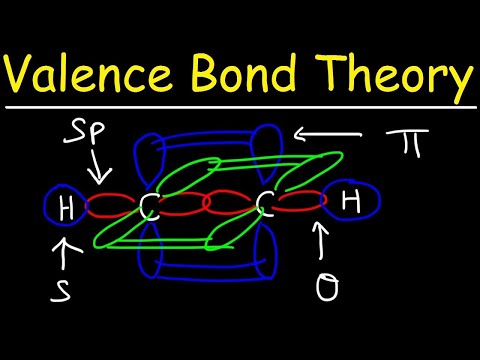
Decoding the Valence Bond Theory 📜
Visualization of 2S and 2P Electrons 🔍
Introducing Hybridization: Linus Pauling's groundbreaking contribution 🌟
Demystifying SP, SP2, and SP3 Hybridizations 📐
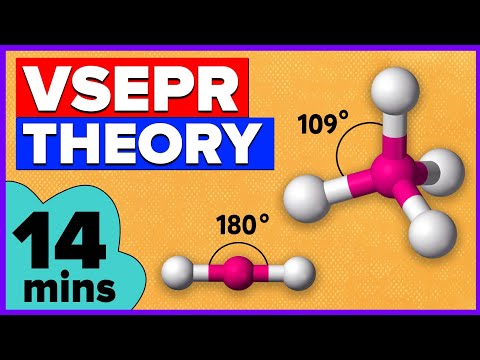
VSEPR Theory & Molecular Shapes Explained 🛠️
Real-life Application: Understanding bond angles in organic chemistry and how they shape molecular geometries 🌐
Easy Hybridization Trick: A simplified approach to deducing molecular geometry! 🧠💡
Join us on this enlightening journey and enrich your understanding of the fundamental concepts that underpin molecular chemistry. Perfect for students, educators, or anyone with a passion for science! Don't forget to like, share, and subscribe for more insightful content.
00:00 VSEPR Theory
03:56 Hybridization
07:11 VSEPR theory for sp3 hybridization
11:03 sp2 hybridization
12:56 sp hybridization
14:16 Hybridization trick
16:03 Examples
17:03 Bond-Line Structures
🧪 More tutorials, practice questions, and organic chemistry workbooks 🧪
👋 Connect with me on social media:
Key Highlights:
Carbon Atom Electron Configuration 🧪
Decoding the Valence Bond Theory 📜
Visualization of 2S and 2P Electrons 🔍
Introducing Hybridization: Linus Pauling's groundbreaking contribution 🌟
Demystifying SP, SP2, and SP3 Hybridizations 📐
VSEPR Theory & Molecular Shapes Explained 🛠️
Real-life Application: Understanding bond angles in organic chemistry and how they shape molecular geometries 🌐
Easy Hybridization Trick: A simplified approach to deducing molecular geometry! 🧠💡
Join us on this enlightening journey and enrich your understanding of the fundamental concepts that underpin molecular chemistry. Perfect for students, educators, or anyone with a passion for science! Don't forget to like, share, and subscribe for more insightful content.
00:00 VSEPR Theory
03:56 Hybridization
07:11 VSEPR theory for sp3 hybridization
11:03 sp2 hybridization
12:56 sp hybridization
14:16 Hybridization trick
16:03 Examples
17:03 Bond-Line Structures
🧪 More tutorials, practice questions, and organic chemistry workbooks 🧪
👋 Connect with me on social media:
Комментарии
 0:06:31
0:06:31
 0:19:32
0:19:32
 0:13:10
0:13:10
 0:10:55
0:10:55
 0:02:25
0:02:25
 0:00:45
0:00:45
 0:07:54
0:07:54
 0:00:16
0:00:16
 0:00:16
0:00:16
 0:50:43
0:50:43
 0:00:13
0:00:13
 0:10:23
0:10:23
 0:14:04
0:14:04
 0:10:39
0:10:39
 0:13:23
0:13:23
 0:11:58
0:11:58
 0:13:27
0:13:27
 0:00:40
0:00:40
 1:16:48
1:16:48
 0:13:48
0:13:48
 0:14:58
0:14:58
 0:20:56
0:20:56
 0:08:39
0:08:39
 0:00:10
0:00:10