filmov
tv
sp3, sp2, sp hybridization for DUMMIES

Показать описание
Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3
How to determine Hybridization - s, sp, sp2, and sp3 - Organic Chemistry
Hybridization of Atomic Orbitals | SP, SP2, SP3 Hybridization of Carbon
Hybrid Orbitals explained - Valence Bond Theory | Orbital Hybridization sp3 sp2 sp
sp3, sp2, sp hybridization for DUMMIES
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
How to identify hybridization of carbon atom|| sp || sp2|| sp3
How to Determine the Hybridization of an Atom (sp, sp2, sp3, sp3d, sp3d2) Practice Problem & Exa...
Sp,Sp2 Hybridization
EASY Method to Find the Hybridization of an Atom | Chemistry |
Orbitals: Crash Course Chemistry #25
Sp3 sp2 sp Hybridization Organic Chemistry Review
Shortcut for Sp3 Sp2 Sp Hybridization
9.3 Hybridization | General Chemistry
Find Hybridization in 60 Seconds! (s, sp, sp2, sp3)
ATOMES ET LIAISONS CHIMIQUES V11: HYBRIDATION DES ORBITALES ATOMIQUES (SP3, SP2, SP)
HIBRIDACIÓN Sp, Sp2, Sp3 DEL CARBONO (QUÍMICA ORGÁNICA)
Sp3 Sp2 Sp Hybridization Made Easy! Part 1 - Organic Chemistry
Identify Hybridization (sp, sp2, sp3)
LES HYBRIDATIONS : ⚛️ Sp, Sp2, Sp3, Sp3d, Sp3d2 ! Comment ça marche ?
Introduction to Electron Orbital Hybridization (sp3 sp2 & sp Made Super Simple!) Organic Chemist...
Hybridization of Atomic Orbitals (sp3, sp2 & sp Hybridization)
Formal Charge and Hybridization On Any Atom (SP, SP2, SP3)
Orbital Hybridization sp3 sp2 sp sigma
Комментарии
 0:10:55
0:10:55
 0:08:22
0:08:22
 0:13:48
0:13:48
 0:11:58
0:11:58
 0:00:45
0:00:45
 0:07:54
0:07:54
 0:05:35
0:05:35
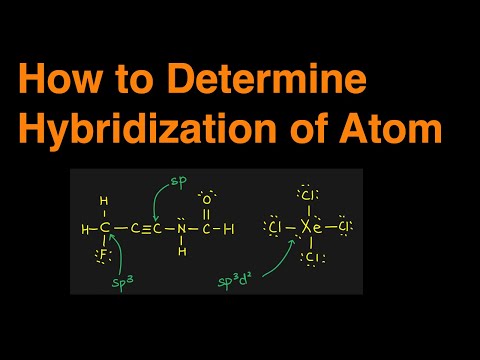 0:03:35
0:03:35
 0:01:08
0:01:08
 0:04:08
0:04:08
 0:10:52
0:10:52
 0:00:40
0:00:40
 0:00:36
0:00:36
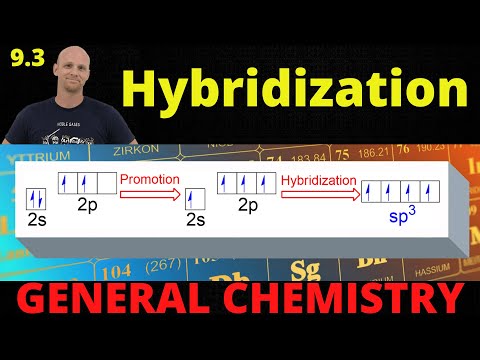 0:16:52
0:16:52
 0:01:00
0:01:00
 0:10:54
0:10:54
 0:07:31
0:07:31
 0:08:03
0:08:03
 0:08:39
0:08:39
 0:11:02
0:11:02
 0:27:32
0:27:32
 0:08:58
0:08:58
 0:05:11
0:05:11
 0:19:19
0:19:19