filmov
tv
Molecular Geometry & VSEPR Theory - Basic Introduction
Показать описание
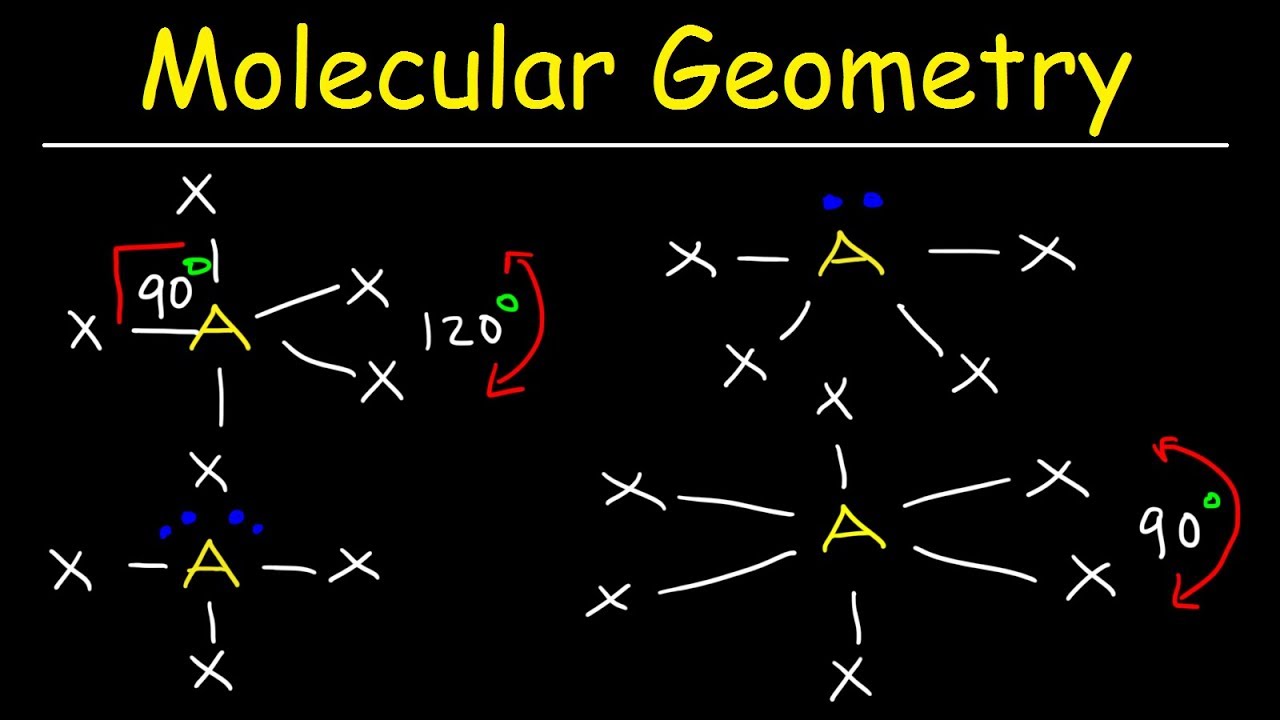
This chemistry video tutorial provides a basic introduction into molecular geometry and vsepr theory. Examples and practice problems include the trigonomal bypiramidal structure, octahedral molecular structure, seesaw, t-shape, square planar, and square pyramidal molecular geometry as well as some of the bond angles of these structures.
How To Draw Lewis Structures:
VSEPR Theory:
Molecular Geometry:
Lewis Dot Structures:
Lewis Structures of Ionic Compounds:
_________________________________
Octet Rule Exceptions:
Resonance Structures:
Polar and Nonpolar Molecules:
Formal Charge Calculations:
Lewis Structures - Mega Review:
________________________________
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Dipole Dipole Forces of Attraction:
Hydrogen Bonding:
Unit Cell Chemistry:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
How To Draw Lewis Structures:
VSEPR Theory:
Molecular Geometry:
Lewis Dot Structures:
Lewis Structures of Ionic Compounds:
_________________________________
Octet Rule Exceptions:
Resonance Structures:
Polar and Nonpolar Molecules:
Formal Charge Calculations:
Lewis Structures - Mega Review:
________________________________
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Dipole Dipole Forces of Attraction:
Hydrogen Bonding:
Unit Cell Chemistry:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:06:31
0:06:31
 0:10:23
0:10:23
 0:13:10
0:13:10
 0:13:23
0:13:23
 0:11:01
0:11:01
 0:05:38
0:05:38
 0:33:39
0:33:39
 0:06:35
0:06:35
 0:01:01
0:01:01
 0:45:18
0:45:18
 0:11:16
0:11:16
 0:08:39
0:08:39
 0:14:58
0:14:58
 0:14:04
0:14:04
 0:06:16
0:06:16
 0:12:36
0:12:36
 0:16:25
0:16:25
 0:52:53
0:52:53
 0:19:11
0:19:11
 0:12:48
0:12:48
 0:04:52
0:04:52
 0:09:57
0:09:57
 0:16:31
0:16:31
 0:07:31
0:07:31