filmov
tv
Atmospheric Pressure and Boiling

Показать описание
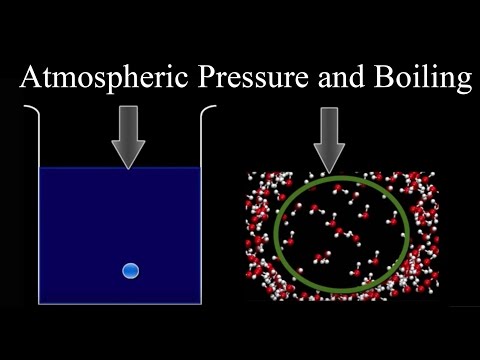
The pressure of gas above a liquid affects the boiling point. In an open system this is called atmospheric pressure. The greater the pressure, the more energy required for liquids to boil, and the higher the boiling point.
Higher Pressure = More Energy Required to Boil = Higher Boiling Point
In an open system this can be visualized as air molecules colliding with the surface of the liquid and creating pressure. This pressure is transmitted throughout the liquid and makes it more difficult for bubbles to form and for boiling to take place.
If the pressure is reduced, the liquid requires less energy to change to a gaseous phase, and boiling occurs at a lower temperature.
Higher Pressure = More Energy Required to Boil = Higher Boiling Point
In an open system this can be visualized as air molecules colliding with the surface of the liquid and creating pressure. This pressure is transmitted throughout the liquid and makes it more difficult for bubbles to form and for boiling to take place.
If the pressure is reduced, the liquid requires less energy to change to a gaseous phase, and boiling occurs at a lower temperature.
Atmospheric Pressure and Boiling
Cooling down water by BOILING it
Boiling, Atmospheric Pressure, and Vapor Pressure
Vapor Pressure and Boiling
Making Water Boil without Heating It
Demonstrating atmospheric pressure 💨🧪 #science #physics #scienceexperiment #sciencefacts
Pressure And Boiling Point w/ Neil deGrasse Tyson
How does air pressure affect the boiling point of water?
Matter In Our Surrounding | PART 3 | CLASS 9th SCIENCE | NCERT | CBSE
Boiling Water Without Heat | Earth Science
Crushing a Coke Can with Ice Water #science #demo #experiment
Can Water Boil by Removing Air? #shorts #science
Atmospheric Pressure & Boiling Point
Introduction to Pressure - Force & Area, Units, Atmospheric Gases, Elevation & Boiling Point
Boiling Water Without Fire?! Science Unlocked 🔥💧 #experiments #physics #funwithphysics #coolscience...
CRUSH huge drums with atmospheric pressure! 🛢️✊ #shorts #science
The boiling point of water and atmospheric pressure.😲🤨(Neil deGrasse Tyson)
How To Boil Water WITHOUT Heat!
What is Vapour Pressure #shorts #chemistry #neet #jee
Vacuum Chamber Experiment: Observe Water Boiling at Room Temperature
Boiling Water with a Vacuum 😲 #shorts #science #stem
'Why does WATER'S BOILING POINT vary?' 🌡️🤔 /w Neil deGrasse Tyson #shorts
How Air Pressure Affects Boiling Water!
vapor pressure explained
Комментарии
 0:02:53
0:02:53
 0:00:56
0:00:56
 0:06:05
0:06:05
 0:01:54
0:01:54
 0:00:37
0:00:37
 0:00:30
0:00:30
 0:00:58
0:00:58
 0:00:41
0:00:41
 0:58:06
0:58:06
 0:02:58
0:02:58
 0:00:24
0:00:24
 0:00:22
0:00:22
 0:03:07
0:03:07
 0:22:48
0:22:48
 0:00:28
0:00:28
 0:00:59
0:00:59
 0:00:34
0:00:34
 0:00:35
0:00:35
 0:00:59
0:00:59
 0:00:57
0:00:57
 0:00:34
0:00:34
 0:00:17
0:00:17
 0:03:23
0:03:23
 0:02:05
0:02:05