filmov
tv
Acid and Base Definitions | Arrhenius, Bronsted-Lowry, and Lewis

Показать описание
What is the definition of an Arrhenius acid or a Lewis base or even a Bronsted-Lowry acid or base? The answer awaits you.
Thanks for stopping by, this is 2 minute classroom and today we are going a little deeper into acids and bases by looking at three different definitions. Arrhenius, Bronsted-Lowry, and Lewis.
The arrhenius definition is the probably the most familiar to you. An arrhenius acid is a molecule that will donate a hydrogen ion (or proton) when dissolved in water. We call it a proton because a typical hydrogen atom has only one proton and one electron, so if you make it a cation by removing the electron, all you have is a proton.
An arrhenius base is a molecule that will produce a hydroxide ion when dissolved in water.
So, the acid provides a proton, and the base provides a hydroxide. However, this is only when dissolved in water.
That’s where the other definitions come in.
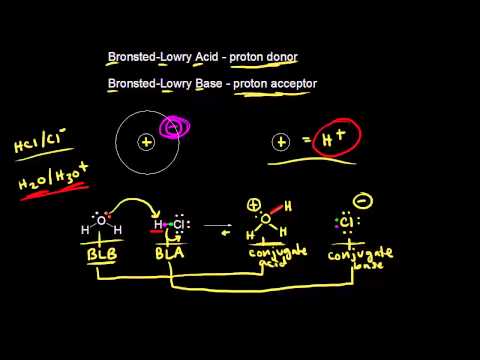
With the Bronsted-Lowry definition, we only refer to what happens to the hydrogen ion or proton. So the acid is a proton donor, much like an arrhenius acid, but the base is defined as a proton acceptor. So if there if a compound will bond a free proton, it is a Bronsted-Lowry base. And unlike Arrhenius acids and bases, this doesn’t have to be in water.
The Lewis definition of acids and bases dives into organic chemistry looking at the electrons of each atom. So a Lewis acid is a molecule that will accept a pair of electrons while a Lewis base is a molecule that donates a pair of electrons.
It’s important to note that these definitions are just different ways to look at and understand acids and bases. Just because a compound can be defined as an arrhenius acid, for example, doesn’t mean it can’t also be defined as a Bronsted-Lowry or Lewis acid.
I hope this video helped you understand these different definitions a little better. If you want to know more about the properties of acids and bases you can watch my acids and bases video.
If you have any questions throw them in the comments, and I’ll catch you next time.
Thanks for stopping by, this is 2 minute classroom and today we are going a little deeper into acids and bases by looking at three different definitions. Arrhenius, Bronsted-Lowry, and Lewis.
The arrhenius definition is the probably the most familiar to you. An arrhenius acid is a molecule that will donate a hydrogen ion (or proton) when dissolved in water. We call it a proton because a typical hydrogen atom has only one proton and one electron, so if you make it a cation by removing the electron, all you have is a proton.
An arrhenius base is a molecule that will produce a hydroxide ion when dissolved in water.
So, the acid provides a proton, and the base provides a hydroxide. However, this is only when dissolved in water.
That’s where the other definitions come in.
With the Bronsted-Lowry definition, we only refer to what happens to the hydrogen ion or proton. So the acid is a proton donor, much like an arrhenius acid, but the base is defined as a proton acceptor. So if there if a compound will bond a free proton, it is a Bronsted-Lowry base. And unlike Arrhenius acids and bases, this doesn’t have to be in water.
The Lewis definition of acids and bases dives into organic chemistry looking at the electrons of each atom. So a Lewis acid is a molecule that will accept a pair of electrons while a Lewis base is a molecule that donates a pair of electrons.
It’s important to note that these definitions are just different ways to look at and understand acids and bases. Just because a compound can be defined as an arrhenius acid, for example, doesn’t mean it can’t also be defined as a Bronsted-Lowry or Lewis acid.
I hope this video helped you understand these different definitions a little better. If you want to know more about the properties of acids and bases you can watch my acids and bases video.
If you have any questions throw them in the comments, and I’ll catch you next time.
Комментарии
 0:02:06
0:02:06
 0:07:49
0:07:49
 0:07:49
0:07:49
 0:09:01
0:09:01
 0:08:54
0:08:54
 0:04:37
0:04:37
 0:08:32
0:08:32
 0:02:43
0:02:43
 0:03:13
0:03:13
 0:33:17
0:33:17
 0:05:24
0:05:24
 0:03:56
0:03:56
 0:06:35
0:06:35
 0:02:30
0:02:30
 0:08:31
0:08:31
 0:11:04
0:11:04
 0:13:12
0:13:12
 0:01:28
0:01:28
 0:11:52
0:11:52
 0:12:51
0:12:51
 0:19:12
0:19:12
 0:09:42
0:09:42
 0:08:18
0:08:18
 0:08:56
0:08:56