filmov
tv
Structure of Atom

Показать описание
Did you know that the Structure of an Atom is just like a Fidget Spinner? Get a visual feel of the Atomic Structure! Rutherford's Gold Foil Experiment (Alpha Scattering Experiment) is demonstrated. Thomson's and Rutherford's Atomic Models are discussed.
Solutions of Top 3 Questions in this video: Coming Soon !!
Try solving the Top 3 Questions! The questions are shown at the end of the video.
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!
Solutions of Top 3 Questions in this video: Coming Soon !!
Try solving the Top 3 Questions! The questions are shown at the end of the video.
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!
GCSE Physics - Atomic Structure, Isotopes & Electrons Shells #32
Atomic Structure And Electrons - Structure Of An Atom - What Are Atoms - Neutrons Protons Electrons
Chemistry - Atomic Structure - EXPLAINED!
The Basic Structure of the Atom | Chemistry and Our Universe: How it All Works
Basic Atomic Structure: A Look Inside the Atom
Atom Explained in Simple Terms
Structure of an Atom
What is an Atom ?
chemistry class 11 structure of atom hand written note #neet
Structure of Atom
Atomic Structure: Protons, Electrons & Neutrons | Chemistry
What is an atom | Matter | Physics | FuseSchool
Plus One Chemistry | Structure of Atom | Full Chapter Revision | Chapter 2 | EXAM WINNER +1
2. Atomic Structure
What Is An Atom? | The Dr. Binocs Show | Best Learning Videos For Kids | Peekaboo Kidz
STRUCTURE OF ATOM in One Shot - From Zero to Hero || Class 9th
Atomic Structure In Just 14 Minutes! REVISION - Super Quick ! JEE & NEET Chemistry | Pahul Sir
Plus One Chemistry - Structure of Atom | One Shot Revision | Xylem Plus One
structure of atom class 11 all formulas
How Small Is An Atom? Spoiler: Very Small.
Structure of Atom Class 11 Chemistry Chapter 2 One Shot | New NCERT CBSE
Class 9 Chemistry Chapter 4| Bohr's Model of the Atom - Structure of the Atom
Electrons, Protons and Neutrons | Chapter 4 | Structure Of Atom | Class 9 Science
What's Inside an Atom? Protons, Electrons, and Neutrons!
Комментарии
 0:05:22
0:05:22
 0:02:20
0:02:20
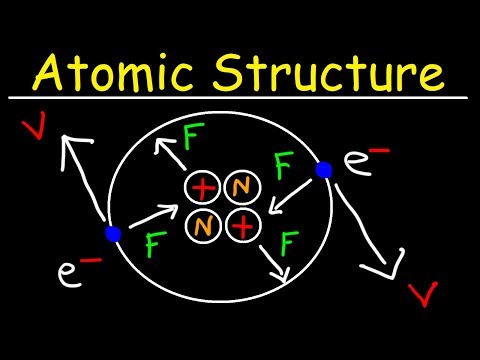 0:11:45
0:11:45
 0:30:31
0:30:31
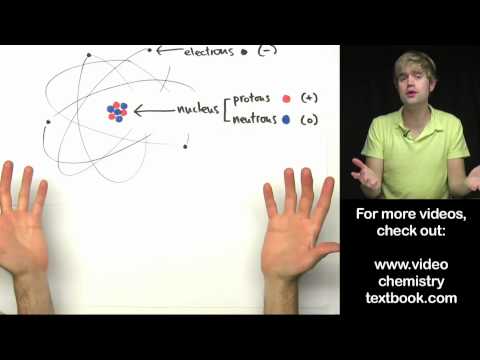 0:07:44
0:07:44
 0:01:44
0:01:44
 0:02:02
0:02:02
 0:09:49
0:09:49
 0:00:20
0:00:20
 0:10:37
0:10:37
 0:07:02
0:07:02
 0:04:00
0:04:00
 1:57:06
1:57:06
 0:39:00
0:39:00
 0:07:17
0:07:17
 1:45:24
1:45:24
 0:13:18
0:13:18
 2:32:00
2:32:00
 0:00:12
0:00:12
 0:04:58
0:04:58
 5:13:38
5:13:38
 0:40:35
0:40:35
 0:09:30
0:09:30
 0:04:06
0:04:06