filmov
tv
What is an Atom ?

Показать описание
What is an Atom?
An atom is the basic unit of matter, and it is the smallest unit of an element that retains the chemical properties of that element. Atoms are the building blocks of all matter in the universe. They are composed of three main subatomic particles:
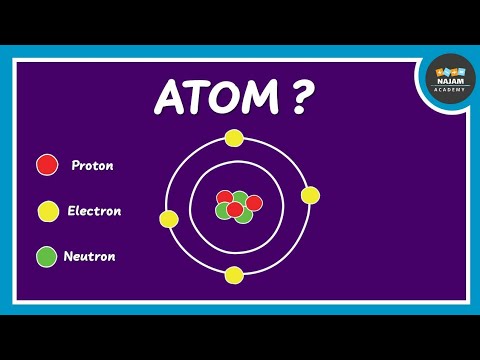
1. Protons: Positively charged particles found in the nucleus of the atom.
2. Neutrons: Neutrally charged particles also located in the nucleus.
3. Electrons: Negatively charged particles that orbit the nucleus in electron shells or energy levels.
The protons and neutrons are tightly packed in the central nucleus of the atom, while the electrons move in various energy levels or orbitals around the nucleus. The number of protons in an atom's nucleus defines its atomic number, which, in turn, determines the element to which the atom belongs. The number of electrons typically matches the number of protons, creating a neutral atom.
Atoms can combine to form molecules through chemical bonding, and they can participate in chemical reactions by gaining, losing, or sharing electrons. The study of atoms and their interactions is a fundamental concept in chemistry and physics and has led to a deeper understanding of the properties and behavior of matter.
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!
An atom is the basic unit of matter, and it is the smallest unit of an element that retains the chemical properties of that element. Atoms are the building blocks of all matter in the universe. They are composed of three main subatomic particles:
1. Protons: Positively charged particles found in the nucleus of the atom.
2. Neutrons: Neutrally charged particles also located in the nucleus.
3. Electrons: Negatively charged particles that orbit the nucleus in electron shells or energy levels.
The protons and neutrons are tightly packed in the central nucleus of the atom, while the electrons move in various energy levels or orbitals around the nucleus. The number of protons in an atom's nucleus defines its atomic number, which, in turn, determines the element to which the atom belongs. The number of electrons typically matches the number of protons, creating a neutral atom.
Atoms can combine to form molecules through chemical bonding, and they can participate in chemical reactions by gaining, losing, or sharing electrons. The study of atoms and their interactions is a fundamental concept in chemistry and physics and has led to a deeper understanding of the properties and behavior of matter.
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!
Комментарии
 0:12:15
0:12:15
 0:04:00
0:04:00
 0:09:49
0:09:49
 0:04:58
0:04:58
 0:07:17
0:07:17
 0:01:44
0:01:44
 0:05:28
0:05:28
 0:06:45
0:06:45
 0:37:26
0:37:26
 0:45:42
0:45:42
 0:04:52
0:04:52
 0:04:06
0:04:06
 0:00:48
0:00:48
 0:01:36
0:01:36
 0:04:42
0:04:42
 0:10:59
0:10:59
 0:03:26
0:03:26
 0:05:52
0:05:52
 0:05:35
0:05:35
 0:05:23
0:05:23
 0:02:56
0:02:56
 0:01:58
0:01:58
 0:04:23
0:04:23
 0:06:43
0:06:43