filmov
tv
2. Atomic Structure

Показать описание
MIT 5.111 Principles of Chemical Science, Fall 2014
Instructor: Catherine Drennan
The backscattering experiment of Rutherford is recreated in the classroom setting. Ping pong balls are used to represent alpha particles and Styrofoam balls connected to a series of strings represent nuclei in a piece of gold foil.
License: Creative Commons BY-NC-SA
Instructor: Catherine Drennan
The backscattering experiment of Rutherford is recreated in the classroom setting. Ping pong balls are used to represent alpha particles and Styrofoam balls connected to a series of strings represent nuclei in a piece of gold foil.
License: Creative Commons BY-NC-SA
2. Atomic Structure
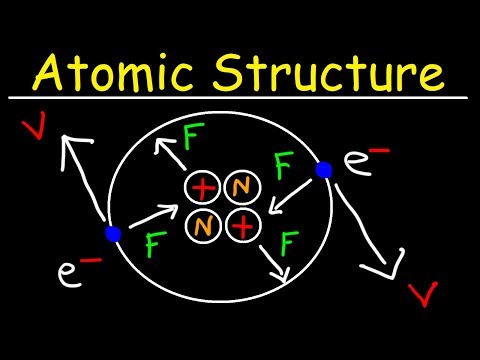
Chemistry - Atomic Structure - EXPLAINED!
GCSE Physics - Atomic Structure, Isotopes & Electrons Shells
Complete Chemistry in 45 Days | Atomic Structure | One Day One Chapter | Akansha Karnwal
Atomic Structure And Electrons - Structure Of An Atom - What Are Atoms - Neutrons Protons Electrons
Atomic Structure Explained (Full Topic) | A Level Physical Chemistry Masterclass
How small are atoms?
Atomic Structure: Protons, Electrons & Neutrons | Chemistry
Atomic Structure Part 2 Short Question Answer #science #class9th #ssc #viralshort #1stgrade #gkgssir
MDCAT I Atomic Structure I Unit 2 I Lec # 1 I Prof. Wajid Ali Kamboh | WAK Entry Test
Class 11 chap 2 | Atomic Structure 02 | Bohr's Atomic ModeL | Most Important For IIT JEE and NE...
2A Atomic Structure - Edexcel IAS Chemistry (Unit 1)
FORM 2: ATOMIC STRUCTURE AND PERIODIC TABLE (Introduction)
Structure of an Atom
Atomic Structure in One Shot | Part 2 | NEET 2025 Chemistry | Anushka Mam
Atomic Structure In Just 14 Minutes! REVISION - Super Quick ! JEE & NEET Chemistry | Pahul Sir
What's Inside an Atom? Protons, Electrons, and Neutrons!
The 2,400-year search for the atom - Theresa Doud
Atomic Structure | A-level Chemistry | OCR, AQA, Edexcel
11 chap 2 : Atomic Structure 01 ||Cathode Rays + Rutherford Alpha Particle Scattering Experiment ||
Quantum Numbers, Atomic Orbitals, and Electron Configurations
IB Chemistry Topic 2 Atomic structure 2.1 The nuclear atom
History of Atomic Theory
Chapter 41: Quantum Mechanics II – Atomic Structure | University Physics (Podcast Summary)
Комментарии
 0:39:00
0:39:00
 0:11:45
0:11:45
 0:05:22
0:05:22
 3:33:39
3:33:39
 0:02:20
0:02:20
 1:14:48
1:14:48
 0:00:48
0:00:48
 0:07:02
0:07:02
 0:00:56
0:00:56
 1:21:17
1:21:17
 1:08:09
1:08:09
 0:44:49
0:44:49
 0:05:05
0:05:05
 0:02:02
0:02:02
 2:45:07
2:45:07
 0:13:18
0:13:18
 0:04:06
0:04:06
 0:05:23
0:05:23
 0:09:16
0:09:16
 1:03:05
1:03:05
 0:08:42
0:08:42
 0:08:14
0:08:14
 0:04:26
0:04:26
 0:16:24
0:16:24