filmov
tv
What is an atom | Matter | Physics | FuseSchool

Показать описание
What is an atom | Matter | Physics | FuseSchool
Atoms are tiny particles that are so small they are not possible to see with the naked eye, and are only barely possible to make out with the most powerful microscopes. Everything that exists in our universe is made up of atoms – including you, I and the device you’re watching this on! In fact, there are about 7 billion billion billion atoms in your body alone.
In this video we are going to look at what atoms are made of, and the mass and size of atoms, and the arrangement of electrons in an atom.Atoms may be tiny particles themselves but they are made of even smaller particles, called subatomic particles.
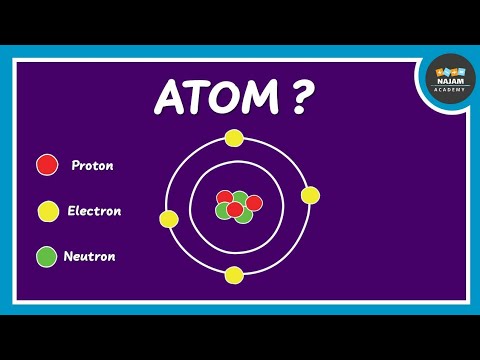
Atoms consist of a central nucleus that contains protons and neutrons. Protons are positively charged (+1) and neutrons have no charge. This makes the nucleus overall positively charged.
Much like how the sun has planets orbiting it, the nucleus has electrons orbiting. Electrons have a negative charge (-1). Because electrons are negatively charged and the nucleus is positively charged, they are electrostatically attracted to each other, like how gravity ensures attraction between planets and the sun.
So that’s the general structure of an atom, what about its mass and size? Protons and neutrons have the same mass, but electrons are so small they weigh almost nothing in comparison to protons and neutrons. In fact they are about 2000 times lighter. As a result, the mass of an atom is concentrated at its nucleus.
So that’s the mass of an atom, but what about it’s size? One way to describe the size of an atom is its radius. The radius of an atoms is the distance from its centre, where the nucleus is, to the outermost shell of electrons. The radius of an atom is typically 10-10m.
So the majority of the mass of an atom is contained in its nucleus, but in terms of size the nucleus is much smaller than the atom as a whole. In fact, electrons can orbit really far away from the nucleus. If the atom was the size of a sports arena, the nucleus could be the size of a pea in the middle!
Let’s finish off by looking at the electrons in a little more detail.
Negatively charged electrons orbit around the positive nucleus in specific orbits or shells. Different atoms have different numbers of shells (or orbitals).
Each shell is of a specific energy level, meaning an electron must possess a certain amount of energy to reside in a certain shell. Electrons in shells closest to the nucleus have the least energy, and electrons in the outermost shells have the highest energy levels.
In other words, electrons orbit the nucleus similar to how planets orbit the sun; however, electrons can only orbit at specific distances and with specific energies.
We will look at the differences in structure of atoms of different elements and the periodic table in this video
So there we have the structure of an atom. Atoms have a positively charged nucleus made up on positive protons and neutral neutrons, which is orbited by negatively charged electrons. The majority of the mass of an atom [10-23g] is found in the nucleus, and the typical size of atoms is 10-10m.
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
These videos can be used in a flipped classroom model or as a revision aid.
Find all of our Chemistry videos here:
Find all of our Biology videos here:
Find all of our Maths videos here:
Atoms are tiny particles that are so small they are not possible to see with the naked eye, and are only barely possible to make out with the most powerful microscopes. Everything that exists in our universe is made up of atoms – including you, I and the device you’re watching this on! In fact, there are about 7 billion billion billion atoms in your body alone.
In this video we are going to look at what atoms are made of, and the mass and size of atoms, and the arrangement of electrons in an atom.Atoms may be tiny particles themselves but they are made of even smaller particles, called subatomic particles.
Atoms consist of a central nucleus that contains protons and neutrons. Protons are positively charged (+1) and neutrons have no charge. This makes the nucleus overall positively charged.
Much like how the sun has planets orbiting it, the nucleus has electrons orbiting. Electrons have a negative charge (-1). Because electrons are negatively charged and the nucleus is positively charged, they are electrostatically attracted to each other, like how gravity ensures attraction between planets and the sun.
So that’s the general structure of an atom, what about its mass and size? Protons and neutrons have the same mass, but electrons are so small they weigh almost nothing in comparison to protons and neutrons. In fact they are about 2000 times lighter. As a result, the mass of an atom is concentrated at its nucleus.
So that’s the mass of an atom, but what about it’s size? One way to describe the size of an atom is its radius. The radius of an atoms is the distance from its centre, where the nucleus is, to the outermost shell of electrons. The radius of an atom is typically 10-10m.
So the majority of the mass of an atom is contained in its nucleus, but in terms of size the nucleus is much smaller than the atom as a whole. In fact, electrons can orbit really far away from the nucleus. If the atom was the size of a sports arena, the nucleus could be the size of a pea in the middle!
Let’s finish off by looking at the electrons in a little more detail.
Negatively charged electrons orbit around the positive nucleus in specific orbits or shells. Different atoms have different numbers of shells (or orbitals).
Each shell is of a specific energy level, meaning an electron must possess a certain amount of energy to reside in a certain shell. Electrons in shells closest to the nucleus have the least energy, and electrons in the outermost shells have the highest energy levels.
In other words, electrons orbit the nucleus similar to how planets orbit the sun; however, electrons can only orbit at specific distances and with specific energies.
We will look at the differences in structure of atoms of different elements and the periodic table in this video
So there we have the structure of an atom. Atoms have a positively charged nucleus made up on positive protons and neutral neutrons, which is orbited by negatively charged electrons. The majority of the mass of an atom [10-23g] is found in the nucleus, and the typical size of atoms is 10-10m.
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
These videos can be used in a flipped classroom model or as a revision aid.
Find all of our Chemistry videos here:
Find all of our Biology videos here:
Find all of our Maths videos here:
Комментарии
 0:04:00
0:04:00
 0:12:15
0:12:15
 0:07:17
0:07:17
 0:01:44
0:01:44
 0:09:49
0:09:49
 0:04:58
0:04:58
 0:06:45
0:06:45
 0:05:28
0:05:28
 0:00:53
0:00:53
 0:04:06
0:04:06
 0:04:52
0:04:52
 0:01:36
0:01:36
 0:05:23
0:05:23
 0:04:42
0:04:42
 0:10:59
0:10:59
 0:05:52
0:05:52
 0:03:02
0:03:02
 0:03:26
0:03:26
 0:01:58
0:01:58
 0:03:00
0:03:00
 0:02:56
0:02:56
 0:07:37
0:07:37
 0:02:12
0:02:12
 0:45:42
0:45:42