filmov
tv
The Shapes of Atomic Orbitals
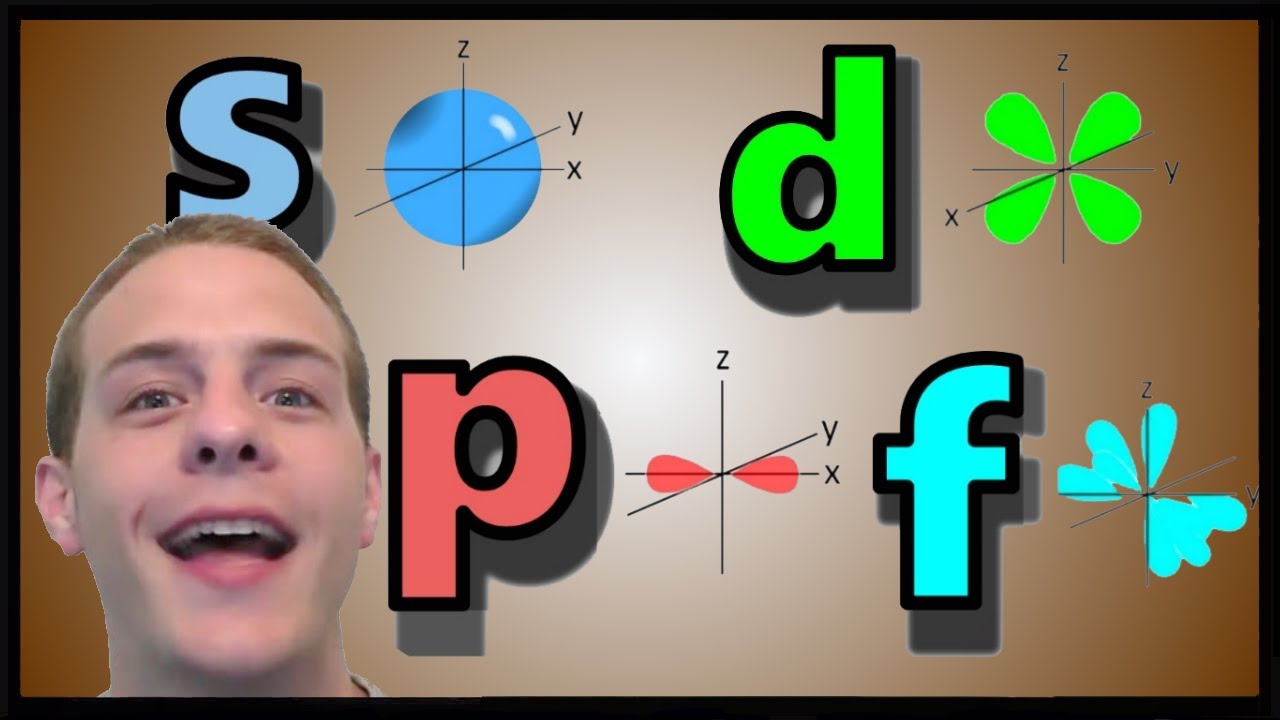
Показать описание
This video is a discussion about the shapes of atomic orbitals, and the scientific principles that govern the shapes of atomic orbitals.
Thanks for watching! 😀
Follow me on:
#Orbitals #QuantumMechanics #Electrons #QuantumMechanicalModel #SchrodingerEquation #WaveFunction #Wave #StandingWave #Node #Antinode #ProbabilityDensity #PauliExclusionPrinciple #Degeneracy #RadialDistributionFunction #QuantumNumbers
Thanks for watching! 😀
Follow me on:
#Orbitals #QuantumMechanics #Electrons #QuantumMechanicalModel #SchrodingerEquation #WaveFunction #Wave #StandingWave #Node #Antinode #ProbabilityDensity #PauliExclusionPrinciple #Degeneracy #RadialDistributionFunction #QuantumNumbers
The Shapes of Atomic Orbitals s-orbital, p-orbital and d-orbital
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
The Shapes of Atomic Orbitals
What ARE atomic orbitals?
Atomic Orbitals Simply Explained! Inorganic CHEM - 1.12
Orbitals, the Basics: Atomic Orbital Tutorial — probability, shapes, energy |Crash Chemistry Academy...
Shapes of atomic orbitals (s,p, d)
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Atomic Orbitals
Atomic orbitals 3D
Orbitals: Crash Course Chemistry #25
A Better Way To Picture Atoms
Grade 11 Chemistry Unit 1: 1.6.3 Shapes of atomic orbitals
Shapes of Atomic Orbitals
Quantum Mechanics: Schrödinger's discovery of the shape of atoms
Atomic Orbitals, Visualized Dynamically
A-Level H2 Chemistry: Shapes of Atomic Orbitals (includes D Orbitals)
shape of the orbitals 👌👌👌👌👌👍👍👍👍
Atomic Orbitals - p, d, f orbitals
EXPLORING THE FASCINATING SHAPES OF ATOMIC ORBITALS IN CHEMISTRY |COMPREHENSIVE GUIDE| #orbitals
Atomic Orbitals and Orbital Shapes
s-orbital || Shapes of orbitals || 11th class chemistry || Ch.no.5
atomic orbitals #orbitals#atom#shorts #youtubeshorts
Chemistry Art: sketching 2p atomic orbitals
Комментарии
 0:10:53
0:10:53
 0:11:19
0:11:19
 0:07:37
0:07:37
 0:21:34
0:21:34
 0:05:56
0:05:56
 0:14:28
0:14:28
 0:00:33
0:00:33
 0:08:42
0:08:42
 0:02:50
0:02:50
 0:05:50
0:05:50
 0:10:52
0:10:52
 0:05:35
0:05:35
 0:12:01
0:12:01
 0:09:44
0:09:44
 0:07:18
0:07:18
 0:08:39
0:08:39
 0:09:34
0:09:34
 0:00:16
0:00:16
 0:06:05
0:06:05
 0:00:59
0:00:59
 0:07:09
0:07:09
 0:06:05
0:06:05
 0:00:15
0:00:15
 0:00:30
0:00:30