filmov
tv
Atomic Orbitals and Orbital Shapes

Показать описание
This video discusses the size and shapes of atomic orbitals, focusing on the s orbital and p orbital, including sub-orbitals and the number of electrons.
The Intro to Orgo series is designed to help incoming organic chemistry students refresh the concepts learned, and perhaps forgotten, from general chemistry.
In this 14 part series I will cover all the basics to help you build a very solid foundation that will help you understand the exciting yet difficult topic that is organic chemistry
Follow my Organic Chemistry Blog for organic chemistry video tutorials, associated practice quizzes, study material and more
You can also find me on
Atomic Orbitals and Orbital Shapes
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
What ARE atomic orbitals?
Atomic Orbitals Simply Explained! Inorganic CHEM - 1.12
Orbitals: Crash Course Chemistry #25
The Shapes of Atomic Orbitals s-orbital, p-orbital and d-orbital
Atomic orbitals 3D
(L3) Atomic orbitals || Shape of atomic orbitals || Orbitals and nodes || Angular nodes #bsc #zchem
Orbitals, the Basics: Atomic Orbital Tutorial — probability, shapes, energy |Crash Chemistry Academy...
Shapes of atomic orbitals (s,p, d)
A Better Way To Picture Atoms
The Shapes of Atomic Orbitals
Atomic Orbitals, Visualized Dynamically
atomic orbitals #orbitals#atom#shorts #youtubeshorts
Atomic Orbitals - p, d, f orbitals
Shells, subshells, and orbitals | Atomic structure and properties | AP Chemistry | Khan Academy
Atomic Orbitals (A-Level IB Chemistry)
Chemistry Art: sketching 2p atomic orbitals
Atomic orbitals explained #shorts #science
Shells, Subshells, and Orbitals - BIOLOGY/CHEMISTRY EP5
What are Shells, Subshells, and Orbitals? | Chemistry
shape of the orbitals 👌👌👌👌👌👍👍👍👍
Shape P Orbital 👍👍
Комментарии
 0:07:09
0:07:09
 0:08:42
0:08:42
 0:11:19
0:11:19
 0:21:34
0:21:34
 0:05:56
0:05:56
 0:10:52
0:10:52
 0:10:53
0:10:53
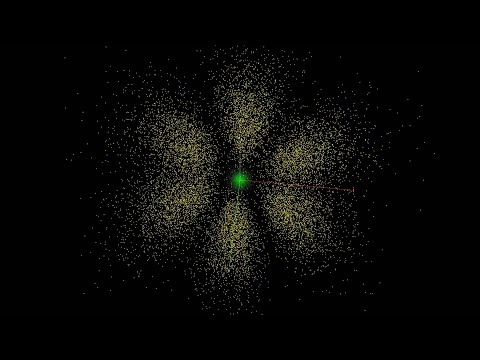 0:05:50
0:05:50
 0:31:29
0:31:29
 0:14:28
0:14:28
 0:00:33
0:00:33
 0:05:35
0:05:35
 0:07:37
0:07:37
 0:08:39
0:08:39
 0:00:15
0:00:15
 0:06:05
0:06:05
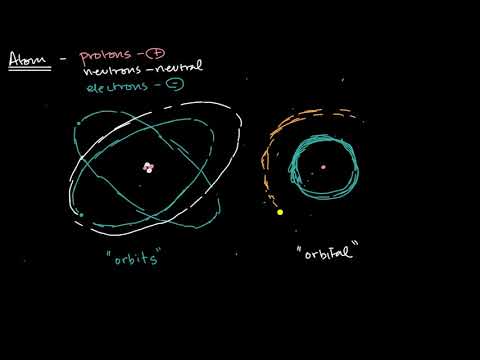 0:09:41
0:09:41
 0:18:49
0:18:49
 0:00:30
0:00:30
 0:00:16
0:00:16
 0:09:23
0:09:23
 0:06:00
0:06:00
 0:00:16
0:00:16
 0:00:16
0:00:16