filmov
tv
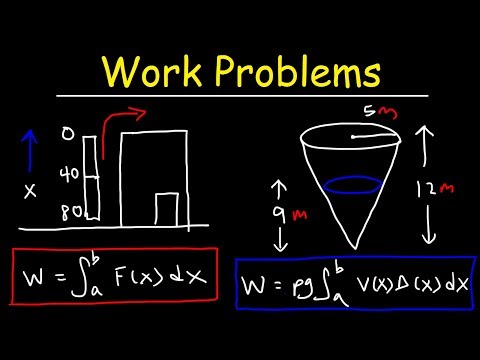
Problem to find Work done if an Expression is given

Показать описание
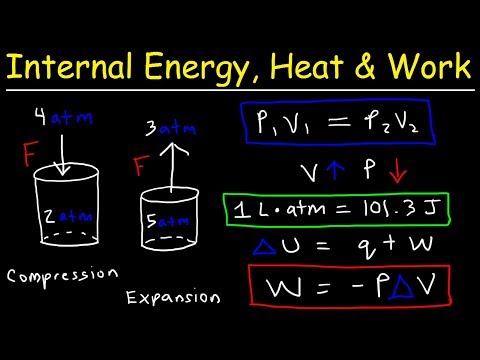
A system of volume V contains a mass m of gas at pressure p and temperature T. The macroscopic properties of the system obey the following relationship
(p + a/V2)(V- b) = mRT
Where a, b, and R are constants.
Obtain an expression for the displacement work done by the system during a constant-temperature expansion from volume V1 to volume V2.
Calculate the work done by a system which contains 10 kg of this gas expanding from 1 m3 to 10 m3 at a temperature of 293 K. Use the values
a = 15.7 ×10 Nm4 , b = 1.07 ×10−2m3 , and R = 0.278 kJ/kg-K.
(p + a/V2)(V- b) = mRT
Where a, b, and R are constants.
Obtain an expression for the displacement work done by the system during a constant-temperature expansion from volume V1 to volume V2.
Calculate the work done by a system which contains 10 kg of this gas expanding from 1 m3 to 10 m3 at a temperature of 293 K. Use the values
a = 15.7 ×10 Nm4 , b = 1.07 ×10−2m3 , and R = 0.278 kJ/kg-K.
 0:09:12
0:09:12
 0:32:06
0:32:06
 0:04:50
0:04:50
 0:11:08
0:11:08
 0:23:29
0:23:29
 0:14:18
0:14:18
 0:07:47
0:07:47
 0:07:12
0:07:12
 0:06:18
0:06:18
 0:07:08
0:07:08
 0:57:10
0:57:10
 0:09:05
0:09:05
 0:04:57
0:04:57
 0:20:45
0:20:45
 0:05:07
0:05:07
 0:00:55
0:00:55
 0:11:53
0:11:53
 0:09:43
0:09:43
 0:06:50
0:06:50
 0:05:36
0:05:36
 0:07:05
0:07:05
 0:14:31
0:14:31
 0:03:17
0:03:17
 0:06:01
0:06:01