filmov
tv
Calculating enthalpy of formation using combustion data

Показать описание
Tutorials of selected topics of IBH chemistry.
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Problems Chemistry
Hess's Law and Heats of Formation
Hess's Law Problems & Enthalpy Change - Chemistry
Enthalpy of formation | Thermodynamics | AP Chemistry | Khan Academy
The EASIEST Method For Solving Hess Cycles
Calculating enthalpy of formation using combustion data
Enthalpies of Formation - Chemsitry Tutorial
Enthalpies of Reactions - Using Average Bond Enthalpies - Chemistry Tutorial
Thermodynamics Chemistry in One Shot || NEET Chemistry || @srichaitanyagosala
Bond Energy Calculations & Enthalpy Change Problems, Basic Introduction, Chemistry
Calculate Standard Enthalpy of Reaction (∆H°rxn) From Standard Heats of Formation (∆H°f) 001
Calculation of enthalpy of formation
How to Calculate Enthalpy of Reaction using Heat of Formation Examples, Practice Problems, Explained
5.1 Standard enthalpy changes of formation and combustion
Enthalpies of Formation
Calculating enthalpy change
[Example] How to Calculate Enthalpy Change of a Reaction.
Hess cycles - combustion
Enthalpy: Crash Course Chemistry #18
CHEM 101 - Using Hess's Law to Calculate Enthalpy Change
Worked example: Using bond enthalpies to calculate enthalpy of reaction | Khan Academy
Using enthalpy to find mass
Born Haber Cycle, Basic Introduction, Lattice Energy, Hess Law & Enthalpy of Formation - Chemist...
Calculating enthalpy change of combustion
Комментарии
 0:16:42
0:16:42
 0:04:58
0:04:58
 0:14:03
0:14:03
 0:09:25
0:09:25
 0:13:46
0:13:46
 0:05:18
0:05:18
 0:06:27
0:06:27
 0:07:49
0:07:49
 0:36:09
0:36:09
 0:11:39
0:11:39
 0:06:41
0:06:41
 0:05:52
0:05:52
 0:05:55
0:05:55
 0:04:26
0:04:26
 0:12:40
0:12:40
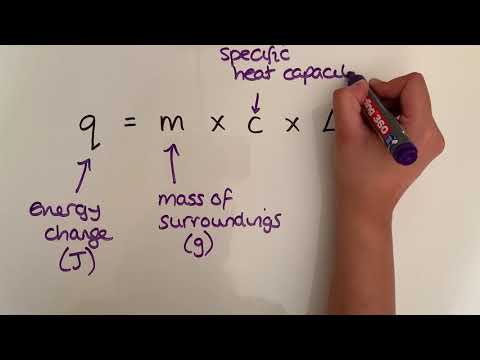 0:07:22
0:07:22
![[Example] How to](https://i.ytimg.com/vi/nmNQUGt6NiM/hqdefault.jpg) 0:01:22
0:01:22
 0:06:39
0:06:39
 0:11:24
0:11:24
 0:04:45
0:04:45
 0:09:03
0:09:03
 0:02:53
0:02:53
 0:10:21
0:10:21
 0:15:26
0:15:26