filmov
tv
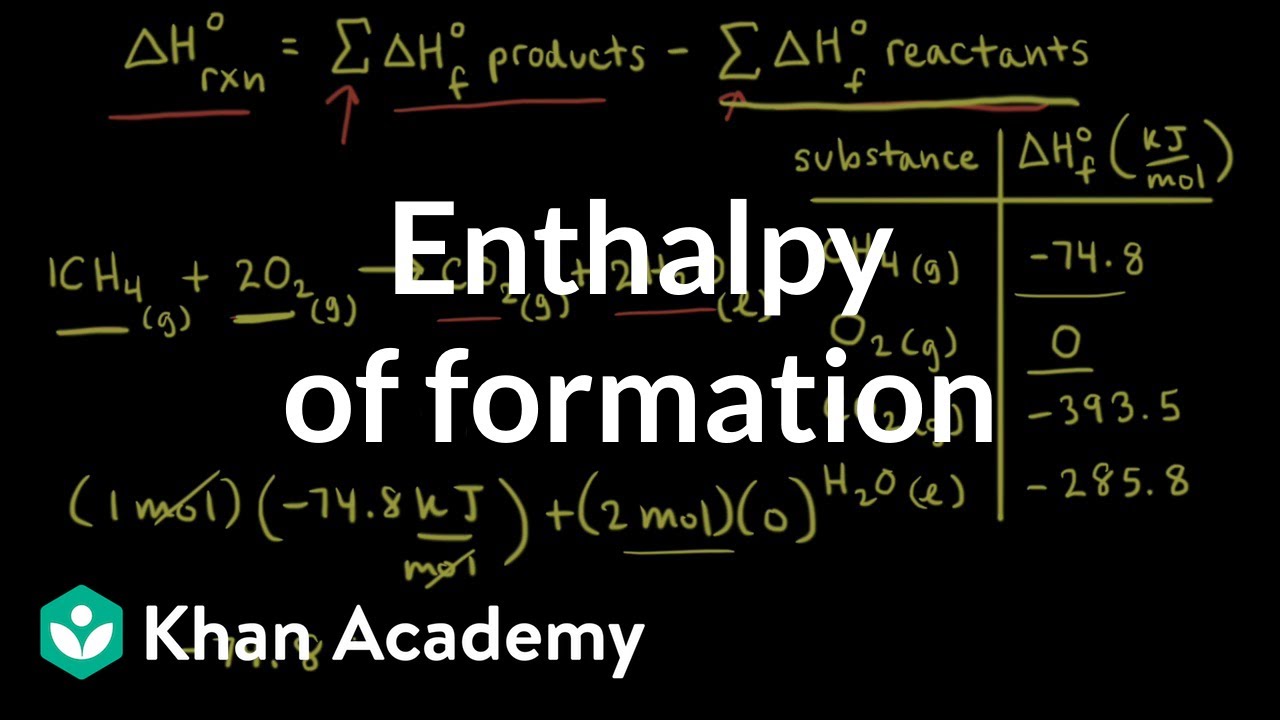
Enthalpy of formation | Thermodynamics | AP Chemistry | Khan Academy
Показать описание
Keep going! Check out the next lesson and practice what you’re learning:
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Enthalpy of formation | Thermodynamics | AP Chemistry | Khan Academy
Hess's Law and Heats of Formation
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Problems Chemistry
Enthalpy of Formation | Enthalpy of Combustion | Thermodynamics
11 chap 6 | Thermodynamics 07 || Heat of Reaction | Enthalpy Of Formation | Enthalpy Of Combustion |
Hess's Law Problems & Enthalpy Change - Chemistry
Tricks to solve Thermochemistry problems easily | Enthalpy of formation combustion
Enthalpy: Crash Course Chemistry #18
Thermodynamics | Theory ✅ Part 1 | NEET | Chemistry | Tamil #anfaz#neettamil#neet2025#neetphysics
Thermochemistry: Heat and Enthalpy
6.6 Standard Enthalpy of Formation and Reaction
Enthalpies of Formation - Chemsitry Tutorial
5.3 Hess's Law and Enthalpy of Formation | General Chemistry
Enthalpy | Thermodynamics
5.1 Standard enthalpy changes of formation and combustion
Thermodynamics: Standard Enthalpy of Formation
What is Enthalpy of Formation?
Mechanical Engineering Thermodynamics - Lec 32, pt 2 of 3: Enthalphy of Formation
Enthalpy of formation | Different enthalpies in chemistry | 11th class chemistry | ch.no.7
15. Thermodynamics: Bond and Reaction Enthalpies
Standard Enthalpy Of Formation - Thermodynamics (Part 17)
MECH351: Chemical Reactions and Combustion/ Enthalpy of formation and enthalpy of combustion
O2 Enthalpy of Formation
Enthalpy Stoichiometry Part 1: Finding Heat and Mass
Комментарии
 0:09:25
0:09:25
 0:04:58
0:04:58
 0:16:42
0:16:42
 0:11:14
0:11:14
 1:10:23
1:10:23
 0:14:03
0:14:03
 0:17:40
0:17:40
 0:11:24
0:11:24
 1:09:26
1:09:26
 0:04:17
0:04:17
 0:17:13
0:17:13
 0:06:27
0:06:27
 0:38:36
0:38:36
 0:10:55
0:10:55
 0:04:26
0:04:26
 0:12:00
0:12:00
 0:08:00
0:08:00
 0:12:02
0:12:02
 0:04:52
0:04:52
 0:38:21
0:38:21
 0:19:25
0:19:25
 0:01:32
0:01:32
 0:00:43
0:00:43
 0:05:50
0:05:50