filmov
tv
Enthalpies of Formation - Chemsitry Tutorial

Показать описание
This chemistry tutorial covers enthalpies of formation, and includes examples of how to calculate the enthalpy change for a reaction using enthalpy of formation values for molecules.
Enthalpies of Formation - Chemsitry Tutorial
Hess's Law and Heats of Formation
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Problems Chemistry
Enthalpy of formation | Thermodynamics | AP Chemistry | Khan Academy
Hess's Law Problems & Enthalpy Change - Chemistry
Enthalpies of Formation
R1.2.3/5.1 Standard Enthalpy Change of Formation and Combustion [HL IB Chemistry 2024]
Enthalpy: Crash Course Chemistry #18
Thermodynamics | Theory ✅ Part 1 | NEET | Chemistry | Tamil #anfaz#neettamil#neet2025#neetphysics
5.3 Hess's Law and Enthalpy of Formation | General Chemistry
The EASIEST Method For Solving Hess Cycles
Heats of Formation
Enthalpy Stoichiometry Part 1: Finding Heat and Mass
6.4 Enthalpy of Chemical Reactions
6.5 Enthalpy of Formation | High School Chemistry
6.6 Standard Enthalpy of Formation and Reaction
Enthalpy of Solution, Enthalpy of Hydration, Lattice Energy and Heat of Formation - Chemistry
IIT/JEE Chemistry Practice #25: Enthalpy of Formation
Enthalpy of reaction | Thermodynamics | AP Chemistry | Khan Academy
Standard Heat of Formation
Standard Enthalpy Changes - combustion and formation (A-Level Chemistry)
Calculating Chemical Reaction Enthalpy From Formation Enthalpies
What is Enthalpy of Formation?
Bond Energy Calculations & Enthalpy Change Problems, Basic Introduction, Chemistry
Комментарии
 0:06:27
0:06:27
 0:04:58
0:04:58
 0:16:42
0:16:42
 0:09:25
0:09:25
 0:14:03
0:14:03
 0:12:40
0:12:40
 0:08:00
0:08:00
 0:11:24
0:11:24
 1:09:26
1:09:26
 0:38:36
0:38:36
 0:13:46
0:13:46
 0:03:32
0:03:32
 0:05:50
0:05:50
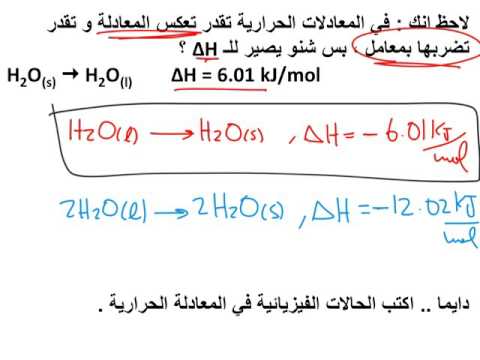 0:13:58
0:13:58
 0:13:27
0:13:27
 0:17:13
0:17:13
 0:16:40
0:16:40
 0:05:33
0:05:33
 0:04:17
0:04:17
 0:11:39
0:11:39
 0:10:17
0:10:17
 0:00:48
0:00:48
 0:08:00
0:08:00
 0:11:39
0:11:39