filmov
tv
5.1 Standard enthalpy changes of formation and combustion

Показать описание
Please note that the standard conditions are now 298 K and 100.0 kPa.
This video covers standard enthalpy changes of formation and combustion.
This video covers standard enthalpy changes of formation and combustion.
5.1 Standard enthalpy changes of formation and combustion
Hess's Law Problems & Enthalpy Change - Chemistry
5.1 Calculating enthalpy changes (SL)
R1.2.3/5.1 Standard Enthalpy Change of Formation and Combustion [HL IB Chemistry 2024]
Enthalpy Changes [IB Chemistry SL/HL]
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Problems Chemistry
[Example] How to Calculate Enthalpy Change of a Reaction.
Calculating enthalpy change
IB Chemistry Topic 5.1: Measuring Energy Changes
Topic 5.1 - What is the Enthalpy change of formation and combustion?
BTEC Applied Science - Unit 5 Chemistry - Standard Enthalpy Changes
Standard enthalpy changes
A LEVEL CHEMISTRY - Standard enthalpy changes - UPDATED FOR 2022
Enthalpy: Crash Course Chemistry #18
IB Chemistry Topic 5 Energetics 5.2 Hess's Law with enthalpy of formation and enthalpy of combu...
Calculating the enthalpy change of a reaction
Standard enthalpy change for combustion | Thermodynamics | Chemistry | Khan Academy
CHEM 101 - Using Hess's Law to Calculate Enthalpy Change
Enthalpy | Thermodynamics
R1.2.3 / R1.2.4 Standard enthalpy change of combustion (HL)
IB 5 1B enthalpy changes & direction of change
[2] F.5 Chemistry Chemical Reactions and Energy (standard enthalpy changes)
5 - 9701_s10_qp_11 (AS) : Energetics : Drawing Hess Cycle
Enthalpy of formation | Thermodynamics | AP Chemistry | Khan Academy
Комментарии
 0:04:26
0:04:26
 0:14:03
0:14:03
 0:08:01
0:08:01
 0:08:00
0:08:00
 0:11:56
0:11:56
 0:16:42
0:16:42
![[Example] How to](https://i.ytimg.com/vi/nmNQUGt6NiM/hqdefault.jpg) 0:01:22
0:01:22
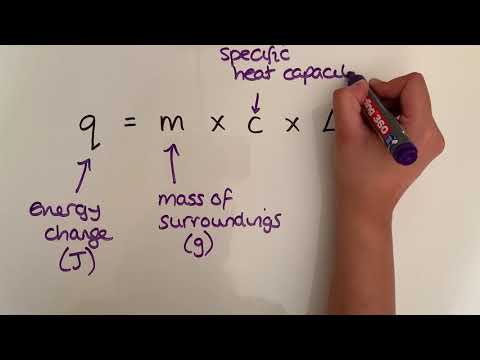 0:07:22
0:07:22
 0:05:52
0:05:52
 0:06:02
0:06:02
 0:16:31
0:16:31
 0:10:14
0:10:14
 0:09:12
0:09:12
 0:11:24
0:11:24
 0:07:40
0:07:40
 0:08:24
0:08:24
 0:07:22
0:07:22
 0:04:45
0:04:45
 0:10:55
0:10:55
 0:03:07
0:03:07
 0:10:02
0:10:02
![[2] F.5 Chemistry](https://i.ytimg.com/vi/ZwsOK_I-ZLk/hqdefault.jpg) 0:38:27
0:38:27
 0:05:36
0:05:36
 0:09:25
0:09:25