filmov
tv
Supercritical water 🔥💧💨

Показать описание
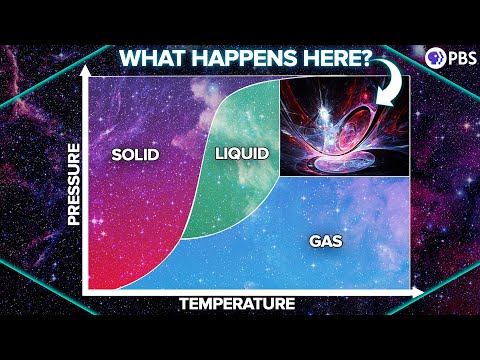
Supercritical water is water that is heated and pressurized beyond its critical point, where it doesn't behave like a typical liquid or gas. The critical point of water is 374°C (705°F) and 22.06 MPa (about 3200 psi). When water reaches this state, it undergoes unique physical changes:
Density: Supercritical water has a density that lies between that of liquid and gas. It is less dense than liquid water but denser than gas.
Viscosity: Its viscosity is lower than liquid water, allowing for faster movement of substances through it.
Solvent Properties: Supercritical water is a powerful solvent. It can dissolve a wide variety of materials, including non-polar substances, which is something liquid water typically cannot do. This makes it useful in chemical reactions and extractions.
Diffusion: Supercritical water has higher diffusion rates than liquid water, allowing for quicker chemical processes.
This state is particularly useful in industries like chemical processing, waste disposal, and energy production. For instance, it’s used in the supercritical water oxidation process to break down hazardous waste into non-toxic substances or in supercritical water gasification to produce hydrogen or biofuels from organic material.
Density: Supercritical water has a density that lies between that of liquid and gas. It is less dense than liquid water but denser than gas.
Viscosity: Its viscosity is lower than liquid water, allowing for faster movement of substances through it.
Solvent Properties: Supercritical water is a powerful solvent. It can dissolve a wide variety of materials, including non-polar substances, which is something liquid water typically cannot do. This makes it useful in chemical reactions and extractions.
Diffusion: Supercritical water has higher diffusion rates than liquid water, allowing for quicker chemical processes.
This state is particularly useful in industries like chemical processing, waste disposal, and energy production. For instance, it’s used in the supercritical water oxidation process to break down hazardous waste into non-toxic substances or in supercritical water gasification to produce hydrogen or biofuels from organic material.
Комментарии
 0:01:00
0:01:00
 0:00:57
0:00:57
 0:09:13
0:09:13
 0:01:00
0:01:00
 0:03:26
0:03:26
 0:08:34
0:08:34
 0:00:56
0:00:56
 0:01:55
0:01:55
 0:02:29
0:02:29
 0:19:53
0:19:53
 0:00:24
0:00:24
 0:09:13
0:09:13
 0:04:43
0:04:43
 0:00:31
0:00:31
 0:06:29
0:06:29
 0:12:14
0:12:14
 0:21:17
0:21:17
 0:10:32
0:10:32
 0:04:33
0:04:33
 0:00:08
0:00:08
 0:04:46
0:04:46
 0:38:52
0:38:52
 0:02:00
0:02:00
 0:10:14
0:10:14