filmov
tv
Burning Forever Chemicals With Water

Показать описание
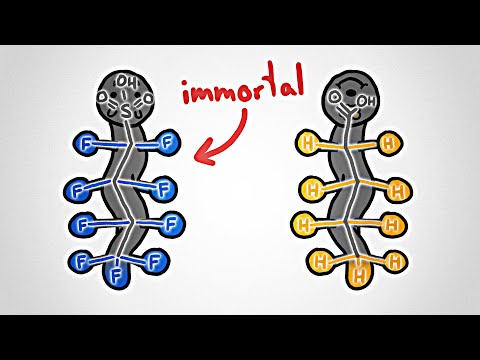
Forever Chemicals, also known as PFAS, are extremely useful industrial chemicals, but they can also leak into the environment, your drinking water, and your blood. And they last (practically) forever. But now chemists have a new way to destroy them: burning them with water.
#chemistry #supercritical #pfas
You might also like other Reactions videos:
You Don't Understand Water (and Neither Does Anyone Else):
The End of Haber Bosch:
Making Drinking Water From Sewage:
The Leidenfrost Effect:
What is Amorphous Ice?
Time to Strike Antifreeze Off Your List of Usable Poisons:
Can We Make Ocean Water Drinkable -- and Should We?
Credits:
Executive Producer:
Matthew Radcliff
Producers:
Elaine Seward
Andrew Sobey
Darren Weaver
Writer:
George Zaidan
Host:
George Zaidan
Scientific Consultants:
Max Krause, Ph.D.
Michelle Boucher, Ph.D.
Leila Duman, Ph.D.
Executive in Charge for PBS: Maribel Lopez
Director of Programming for PBS: Gabrielle Ewing
Assistant Director of Programming for PBS: John Campbell
Reactions is a production of the American Chemical Society.
© 2023 American Chemical Society. All rights reserved.
Sources:
Full article: Developing innovative treatment technologies for PFAS-containing wastes
Supercritical water oxidation: A technical review - Bermejo - 2006 - AIChE Journal - Wiley Online Library
The When and Where of Water in the History of the Universe - ScienceDirect
Properties of supercritical fluids | SpringerLink
Modern Supercritical Fluid Technology for Food Applications | Annual Review of Food Science and Technology
Supercritical Water Oxidation as an Innovative Technology for PFAS Destruction | Journal of Environmental Engineering | Vol 148, No 2
Oxygen (O) and water
Current and Foreseeable Applications of Supercritical Water for Energy and the Environment - Loppinet‐Serani - 2008 - ChemSusChem - Wiley Online Library
Destruction of perfluorooctanesulfonate (PFOS) in a batch supercritical water oxidation reactor ScienceDirect
#chemistry #supercritical #pfas
You might also like other Reactions videos:
You Don't Understand Water (and Neither Does Anyone Else):
The End of Haber Bosch:
Making Drinking Water From Sewage:
The Leidenfrost Effect:
What is Amorphous Ice?
Time to Strike Antifreeze Off Your List of Usable Poisons:
Can We Make Ocean Water Drinkable -- and Should We?
Credits:
Executive Producer:
Matthew Radcliff
Producers:
Elaine Seward
Andrew Sobey
Darren Weaver
Writer:
George Zaidan
Host:
George Zaidan
Scientific Consultants:
Max Krause, Ph.D.
Michelle Boucher, Ph.D.
Leila Duman, Ph.D.
Executive in Charge for PBS: Maribel Lopez
Director of Programming for PBS: Gabrielle Ewing
Assistant Director of Programming for PBS: John Campbell
Reactions is a production of the American Chemical Society.
© 2023 American Chemical Society. All rights reserved.
Sources:
Full article: Developing innovative treatment technologies for PFAS-containing wastes
Supercritical water oxidation: A technical review - Bermejo - 2006 - AIChE Journal - Wiley Online Library
The When and Where of Water in the History of the Universe - ScienceDirect
Properties of supercritical fluids | SpringerLink
Modern Supercritical Fluid Technology for Food Applications | Annual Review of Food Science and Technology
Supercritical Water Oxidation as an Innovative Technology for PFAS Destruction | Journal of Environmental Engineering | Vol 148, No 2
Oxygen (O) and water
Current and Foreseeable Applications of Supercritical Water for Energy and the Environment - Loppinet‐Serani - 2008 - ChemSusChem - Wiley Online Library
Destruction of perfluorooctanesulfonate (PFOS) in a batch supercritical water oxidation reactor ScienceDirect
Комментарии
 0:10:32
0:10:32
 0:06:29
0:06:29
 0:00:55
0:00:55
 0:01:01
0:01:01
 0:03:46
0:03:46
 0:06:07
0:06:07
 0:04:25
0:04:25
 0:00:52
0:00:52
 0:00:33
0:00:33
 0:00:35
0:00:35
 0:01:00
0:01:00
 0:00:29
0:00:29
 0:00:59
0:00:59
 0:00:38
0:00:38
 0:04:42
0:04:42
 0:28:48
0:28:48
 0:12:07
0:12:07
 0:26:25
0:26:25
 0:01:18
0:01:18
 0:03:36
0:03:36
 0:02:16
0:02:16
 0:00:53
0:00:53
 0:02:11
0:02:11
 0:00:54
0:00:54