filmov
tv
Supercritical carbon dioxide (sCO2) | How does it look like?

Показать описание
Liquid CO2 is heated in a pressure vessel and brought into the supercritical state.
CO2 becomes supercitical at 7.4 MPa and ~31°C.
In this experiment, liquid CO2 is enclosed in a pressure vessel. Pressure and temperature are increased by using a heating cartridge, so that the CO2 changes to the supercritical state when the critical point (31°C, 7.4 MPa) is reached.
Afterwards cooling of the pressure vessel is followed by an abrupt re-condensation.
CO2 becomes supercitical at 7.4 MPa and ~31°C.
In this experiment, liquid CO2 is enclosed in a pressure vessel. Pressure and temperature are increased by using a heating cartridge, so that the CO2 changes to the supercritical state when the critical point (31°C, 7.4 MPa) is reached.
Afterwards cooling of the pressure vessel is followed by an abrupt re-condensation.
Sandia’s supercritical carbon dioxide (sCO2) Brayton Cycle
Supercritical carbon dioxide (sCO2) | How does it look like?
How is supercritical carbon dioxide used for energy purposes? Here is the answer
sCO2-4-NPP project
Hanwha Power Systems introduces innovative waste-to-power sCO2 solution
♻️⚡️A Better way to Make Electricity WITH CO2⚡️♻️
sCO2-Flex: Making Electricity Production More Flexible
Table-Sized Turbine
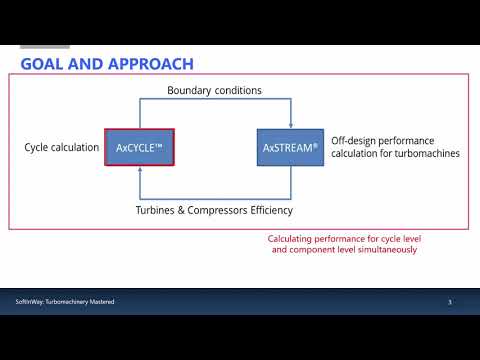
Off-Design Simulation for Supercritical CO2 Power Plants using AxSTREAM
What makes Supercritical Carbon dioxide an exceptional SCFE solvent? | Cybernetik
sCO2-Flex
How To Do Supercritical CO2 Extraction
Episode 22: The Power of sCO2
BREAKTHROUGH Supercritical Turbine Breaks RECORDS
A close look at supercritical carbon dioxide CO2
Supercritical CO2 Turbines Explained {Future Friday Ep92}
sCO2 Webinar 2019
Supercritical CO2 Brayton Power Cycles -Animation
Inauguration of the Supercritical Carbon Dioxide (S-CO2) Brayton Cycle Test Loop Facility
Supercritical CO2! #science #physics #supercritical
Supercritical CO2 Brayton Power Cycles Test facility@indianinstituteofscienceii3263
Net Worth of Geothermal Power Production from Supercritical CO2
how supercritical fluids give you decaf coffee
4h14m11s10f LFTR Carbon Dioxide Gas Turbine - Efficient and Small Scale - TR2016a
Комментарии
 0:02:58
0:02:58
 0:02:00
0:02:00
 0:03:31
0:03:31
 0:04:03
0:04:03
 0:05:06
0:05:06
 0:07:02
0:07:02
 0:05:35
0:05:35
 0:01:13
0:01:13
 0:07:17
0:07:17
 0:00:40
0:00:40
 0:01:47
0:01:47
 0:01:57
0:01:57
 0:33:59
0:33:59
 0:05:29
0:05:29
 0:07:59
0:07:59
 0:16:30
0:16:30
 0:23:22
0:23:22
 0:02:44
0:02:44
 0:31:34
0:31:34
 0:00:16
0:00:16
 0:04:33
0:04:33
 0:01:29
0:01:29
 0:00:56
0:00:56
 0:00:20
0:00:20