filmov
tv
Internal Energy
Показать описание
We know about kinetic energy and potential energy, which can interchange when an object moves through a gravitational field, so let's add to that list the internal energy of the object. This gives us a new and comprehensive way to describe conservation of energy. Check it out!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Internal Energy
GCSE Physics - Internal Energy and Specific Heat Capacity #28
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
Internal Energy
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume, Chemistry Problems
The First Law of Thermodynamics: Internal Energy, Heat, and Work
ENTHALPY and INTERNAL ENERGY in 12 Minutes!
Energy & Chemistry: Crash Course Chemistry #17
Internal Energy of an Ideal Gas - Thermal Physics (Lesson 6)
Introduction to Internal Energy of Solids, Liquids, and Gases
Total Energy and internal energy of a system - Thermodynamics
Internal Energy - GCSE Physics Revision
Internal Energy
12.6 Heat and Internal Energy
Thermodynamics: Internal Energy and Degree of freedom (Animation)
GCSE Physics - Specific Latent Heat #29
Worked example: Internal energy | Thermodynamics | Physics | Khan Academy
Internal Energy Introduction
THERMODYNAMICS -09 || Internal energy of a system.
Relationship Between Energy, Internal Energy, Enthalpy, and Heat
Ideal Gases - Specific Heat, Internal Energy, Enthalpy | Thermodynamics | (Solved Problems)
Enthalpy | Thermodynamics
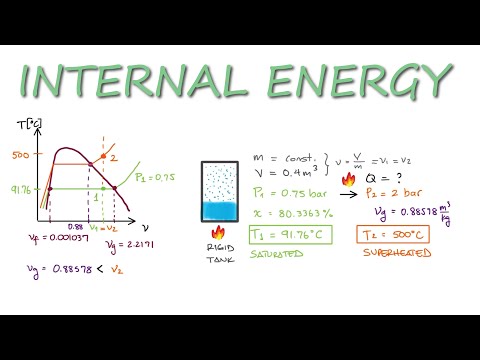
Saturated to Superheated INTERNAL ENERGY Example in 3 Minutes!
What Is Internal Energy In Thermodynamics Class 11 Chemistry By Arvind Arora Sir
Комментарии
 0:03:48
0:03:48
 0:04:36
0:04:36
 0:11:27
0:11:27
 0:06:15
0:06:15
 0:23:29
0:23:29
 0:05:44
0:05:44
 0:11:52
0:11:52
 0:09:26
0:09:26
 0:02:15
0:02:15
 0:03:53
0:03:53
 0:04:35
0:04:35
 0:03:23
0:03:23
 0:06:49
0:06:49
 0:03:07
0:03:07
 0:09:27
0:09:27
 0:06:26
0:06:26
 0:03:29
0:03:29
 0:03:36
0:03:36
 0:15:02
0:15:02
 0:05:57
0:05:57
 0:11:25
0:11:25
 0:10:55
0:10:55
 0:03:37
0:03:37
 0:01:41
0:01:41