filmov
tv
Internal Energy of an Ideal Gas - Thermal Physics (Lesson 6)

Показать описание
Lesson 6
In this video, we use the kinetic energy equation and an assumption of the ideal gas to arrive at the internal energy equation:
Internal energy = 3/2 nRT
Lesson 5
Derivation of the two versions of the mean kinetic energy equation of gas molecules:
KE=3/2NkT
KE=3/2nRT
Lesson 4
Using kinetic theory of gas to derive the equation of gas pressure:
pV=1/3 Nmu^2
Lesson 3
Two versions of the empirical ideal gas equation:
pV = nRT
pV = NkT
Lesson 2
Boyle's law, Charles' law, and Gay-Lussac's law, and the combined gas law
Lesson 1
Describing an Ideal Gas - Moles, Molar Mass, Relative Molar Mass, Avogadro Constant
#physics #kinetictheory #alevelphysics #neetphysics #class11
In this video, we use the kinetic energy equation and an assumption of the ideal gas to arrive at the internal energy equation:
Internal energy = 3/2 nRT
Lesson 5
Derivation of the two versions of the mean kinetic energy equation of gas molecules:
KE=3/2NkT
KE=3/2nRT
Lesson 4
Using kinetic theory of gas to derive the equation of gas pressure:
pV=1/3 Nmu^2
Lesson 3
Two versions of the empirical ideal gas equation:
pV = nRT
pV = NkT
Lesson 2
Boyle's law, Charles' law, and Gay-Lussac's law, and the combined gas law
Lesson 1
Describing an Ideal Gas - Moles, Molar Mass, Relative Molar Mass, Avogadro Constant
#physics #kinetictheory #alevelphysics #neetphysics #class11
Internal Energy of an Ideal Gas - Molar Heat Capacity of Monatomic & Diatomic Gases, Gamma Ratio...
Internal Energy
Internal Energy of an Ideal Gas - Thermal Physics (Lesson 6)
HTPIB15G Total Internal Energy of an Ideal Gas
Ideal Gases - Specific Heat, Internal Energy, Enthalpy | Thermodynamics | (Solved Problems)
The First Law of Thermodynamics: Internal Energy, Heat, and Work
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
ENTHALPY and INTERNAL ENERGY in 12 Minutes!
Internal energy, enthalpy, and specific heats of ideal gas
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume, Chemistry Problems
How do internal energy and enthalpy of an ideal gas depend on temperature
Internal Energy
Chapter 5: Internal energy as a function of temperature and volume
Internal Energy, Ideal Gases: Thermodynamics: Edexcel A-level Physics
Internal energy of an ideal gas depends upon
PV-Diagram Ideal Gas Cycle: Calculate Heat, Work, Change in Internal Energy, and Efficiency
Internal Energy of An Ideal Gas | Heat & Thermodynamics Vid#05(B)
Internal Energy of Ideal gas | YOLO JEE Advance Physics with Vikrant Kirar
Internal Energy of an Ideal Gas
Internal energy of an ideal gas depends on: A. Pressure only B. Volume only C. Temperature only D. N
11.3.5 Derivation of Internal Energy Formula for Ideal Monatomic Gas
If the internal energy of an ideal gas decreases by the same amount as the work done by the syst...
Internal energy & molar heat capacity of ideal gases (derivation, kinetic theory of gases)
Example: Using specific heat to calculate ideal gas internal energy change
Комментарии
 0:10:36
0:10:36
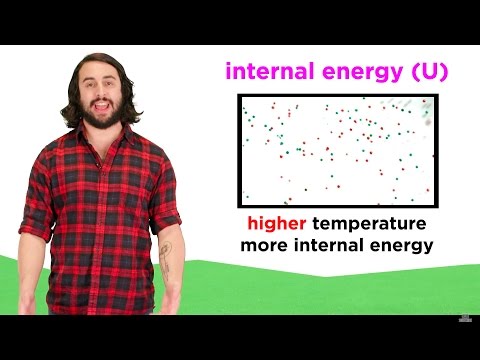 0:03:48
0:03:48
 0:02:15
0:02:15
 0:10:04
0:10:04
 0:11:25
0:11:25
 0:05:44
0:05:44
 0:11:27
0:11:27
 0:11:52
0:11:52
 0:20:21
0:20:21
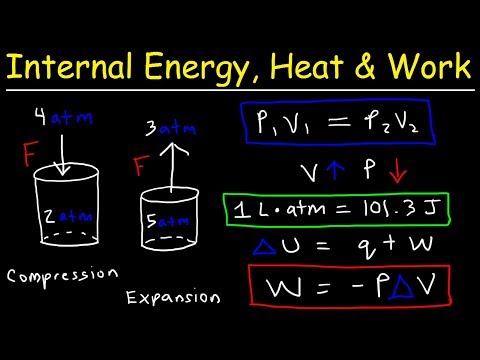 0:23:29
0:23:29
 0:01:51
0:01:51
 0:06:49
0:06:49
 0:09:12
0:09:12
 0:11:45
0:11:45
 0:01:30
0:01:30
 0:16:41
0:16:41
 0:09:38
0:09:38
 0:06:11
0:06:11
 0:02:25
0:02:25
 0:00:17
0:00:17
 0:04:10
0:04:10
 0:01:29
0:01:29
 0:27:09
0:27:09
 0:03:23
0:03:23