filmov
tv
Comparing Melting & Boiling Points of Every Organic Functional Group // HSC Chemistry

Показать описание
⌚Timestamp
📚Syllabus
• explain the properties within and between the homologous series of alkanes with reference to the intermolecular and intramolecular bonding present
• explain the properties within and between the homologous series of alcohols with reference to the intermolecular and intramolecular bonding present
• explain the properties within and between the homologous series of carboxylic acids amines and amides with reference to the intermolecular and intramolecular bonding present
Intermolecular Forces and Boiling Points
Which Compound Has a Higher Boiling Point? Intermolecular Force Boiling Point Relationship, Examples
Ranking by Melting/Boiling Point
Melting and Boiling Points - p98 (Foundation p97)
Ranking Intermolecular Forces - Compare Highest/Lowest Boiling Points with IMF's
Boiling Point of Organic Compounds
Intermolecular Forces - Hydrogen Bonding, Dipole Dipole Interactions - Boiling Point & Solubilit...
Comparing Melting & Boiling Points of Every Organic Functional Group // HSC Chemistry
Ionic Metallic Covalent Boiling Points / Melting Points Explanation based on structure GCSE Chem
Intermolecular Forces and Boiling Point (AP Chemistry)
Melting Point, Boiling Point and Freezing Point | Chemistry
Chemistry - How Boiling and Melting Points are affected by Geometrical Isomers
Lesson 5: Comparison of Melting Points & Boiling Points of Iron Chlorides | Topic: Polarizatio...
Ranking Solubilities and Boiling Points
Intermolecular Forces Trends: Melting & Boiling Point, Viscosity, Surface Tension, Vapor Pressur...
How to rank boiling points from highest to lowest using IMF - Dr K
Boiling point and Melting point-Physical Properties
🔥Comparison of Melting Point 😱Super Trick ✅ in 1 Minute #shorts #organicchemistry #jee #neet
Melting and Boiling - Boiling Point and Melting Point - Learning Junction
Comparing melting and boiling point? | Chemistry students should watch this!
Comparing the Boiling Point of Alkanes || Organic Chemistry
🔥Comparison of Boiling Point in Chemistry | Super Trick #organicchemistry #jee #neet
Intermolecular Forces grade 11: Boiling point
Melting Point Of Different Metals
Комментарии
 0:10:54
0:10:54
 0:05:53
0:05:53
 0:05:04
0:05:04
 0:06:31
0:06:31
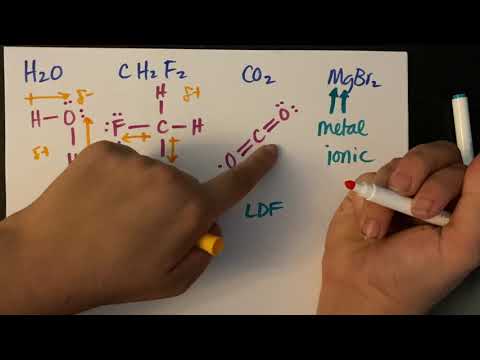 0:09:33
0:09:33
 0:15:42
0:15:42
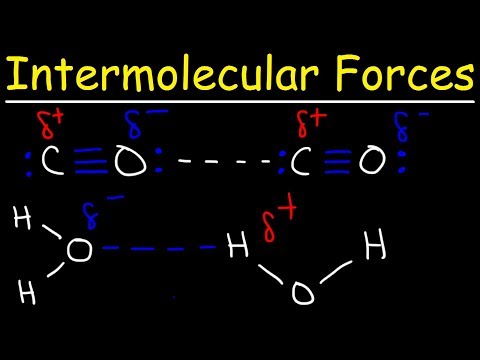 0:10:40
0:10:40
 0:08:05
0:08:05
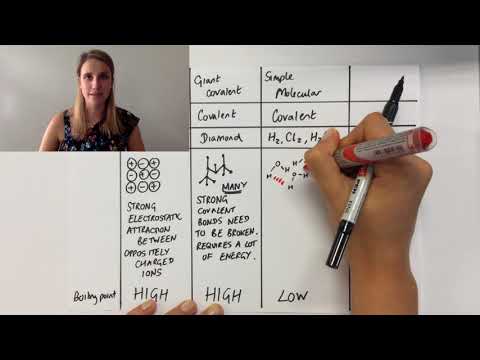 0:06:27
0:06:27
 0:05:20
0:05:20
 0:06:45
0:06:45
 0:02:51
0:02:51
 0:08:32
0:08:32
 0:10:09
0:10:09
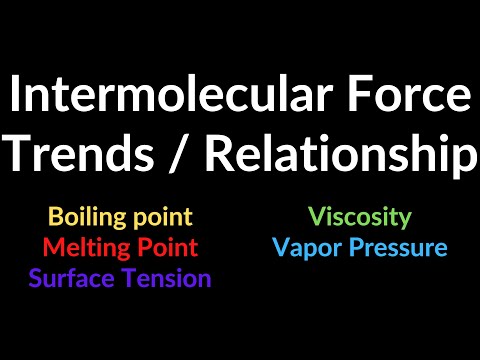 0:02:51
0:02:51
 0:06:02
0:06:02
 0:02:48
0:02:48
 0:00:37
0:00:37
 0:03:42
0:03:42
 0:01:15
0:01:15
 0:10:10
0:10:10
 0:01:00
0:01:00
 0:06:28
0:06:28
 0:02:48
0:02:48