filmov
tv
Ranking Intermolecular Forces - Compare Highest/Lowest Boiling Points with IMF's

Показать описание
Ranking Intermolecular Forces - Compare Highest/Lowest Boiling Points with IMF's
Intermolecular Forces and Boiling Points
Which Compound Has a Higher Boiling Point? Intermolecular Force Boiling Point Relationship, Examples
How to Identify the Intermolecular Force a Compound Has: London Dispersion, Dipole Dipole, H-Bonding
CHEM 112: Comparing/Ranking Intermolecular Forces!
Intermolecular Forces - Hydrogen Bonding, Dipole Dipole Interactions - Boiling Point & Solubilit...
Ranking by Melting/Boiling Point
How to rank boiling points from highest to lowest using IMF - Dr K
Lecture 38 - Fri Nov 22 - AU24
How to identify intermolecular forces?
Intermolecular Forces and Boiling Point (AP Chemistry)
Ranking Solid Intermolecular Forces
Intermolecular Forces Trends: Melting & Boiling Point, Viscosity, Surface Tension, Vapor Pressur...
Solubility and intermolecular forces | AP Chemistry | Khan Academy
How to Determine the Strength of Intermolecular Forces (IMFs) and Rank Boiling Points
Ranking Solubilities and Boiling Points
Rank the compounds in each group in order of increasing strength of intermolecular forces a CH NH ,
1.6 Intermolecular Forces | Organic Chemistry
Ordering relative strength of Van der Waals Force compared to other forces
Acids & Bases - Inductive Effect, Electronegativity, Hybridization, Resonance & Atomic Size
3 main factors affecting polarity #shorts #ochem
Intermolecular Forces 2 5 compare boiling points
Boiling Point of Organic Compounds
Intermolecular Forces and Trends, Formal Charges, Hund's Rule, Lattice Structures and Unit Cell...
Комментарии
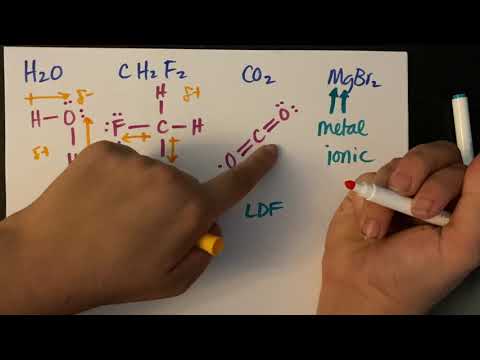 0:09:33
0:09:33
 0:10:54
0:10:54
 0:05:53
0:05:53
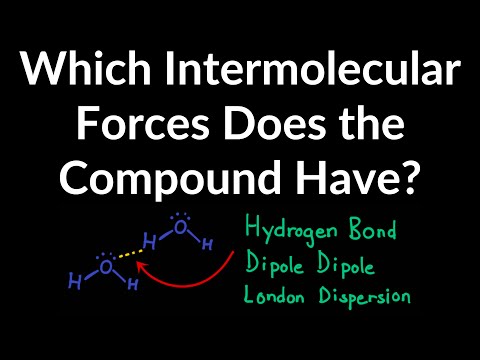 0:05:37
0:05:37
 0:29:51
0:29:51
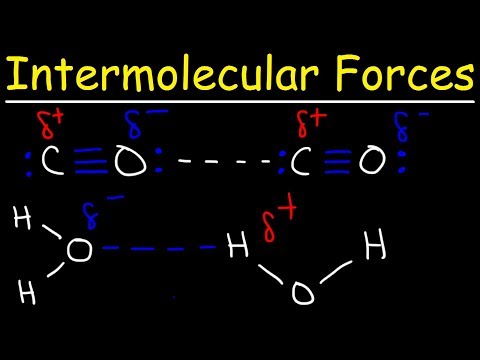 0:10:40
0:10:40
 0:05:04
0:05:04
 0:06:02
0:06:02
 0:55:19
0:55:19
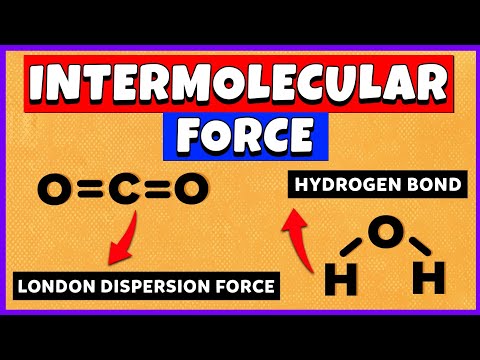 0:08:05
0:08:05
 0:05:20
0:05:20
 0:07:15
0:07:15
 0:02:51
0:02:51
 0:04:23
0:04:23
 0:12:22
0:12:22
 0:10:09
0:10:09
 0:02:14
0:02:14
 0:32:50
0:32:50
 0:02:54
0:02:54
 0:09:19
0:09:19
 0:01:01
0:01:01
 0:06:18
0:06:18
 0:15:42
0:15:42
 0:55:46
0:55:46