filmov
tv
Catalysis in Chemical Reactions - AP Chem Unit 5, Topic 11

Показать описание
In this video, Mr. Krug shows students how to recognize a catalyst in a reaction mechanism and how to work with a catalyst in a rate-determining step when writing a rate law. He then finishes the video with some conceptual review questions about chemical kinetics.
What Are Catalysts? | Reactions | Chemistry | FuseSchool
Energy Diagrams, Catalysts, and Reaction Mechanisms
Chemistry - 3Sec - The effect of catalysts on the rate of chemical reactions
Homogeneous vs Heterogeneous Catalysts - Basic Introduction
Effect of Catalyst on Rate of Reaction (Explained with Potential Energy Diagram)
Catalysis in Chemical Reactions - AP Chem Unit 5, Topic 11
Science in 1 minute: What does a catalyst do?
Demonstration of a Catalyst
Grade 10 Chemistry unit 1 part 4 Chemical Reaction and Stoichiometry
How To Identify The Intermediate & Catalyst In a Reaction Mechanism - Kinetics Chemistry
Catalysts and Homogeneous and Heterogeneous Catalysis (A-Level IB Chemistry)
Introduction to Catalysis and Catalysts // Reactor Engineering - Class 135
How to speed up chemical reactions (and get a date) - Aaron Sams
037-Catalytic Mechanisms
Enzymes - Catalysts
Catalysis
GCSE Chemistry - Factors Affecting the Rate of Reaction #47
Catalyst and Inhibitors
34. Kinetics: Catalysts
What Are Catalytic Converters | Environment | Chemistry | FuseSchool
What is a Catalyst? // Reactor Engineering - Class 137
14.3 Reaction Mechanisms, Catalysts, and Reaction Coordinate Diagrams | General Chemistry
Summary of Steps involved in a Catalytic Reaction // Reactor Engineering - Class 164
Catalysis in Chemical Reactions
Комментарии
 0:03:34
0:03:34
 0:05:23
0:05:23
 0:01:55
0:01:55
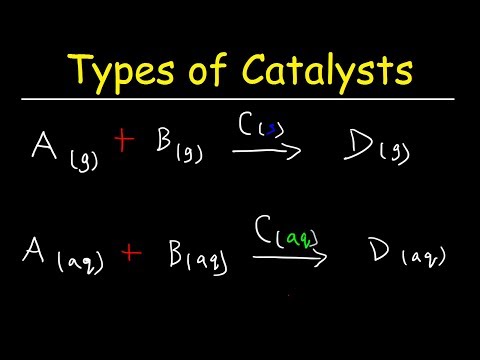 0:01:34
0:01:34
 0:03:37
0:03:37
 0:07:17
0:07:17
 0:01:15
0:01:15
 0:02:21
0:02:21
 0:19:40
0:19:40
 0:05:15
0:05:15
 0:10:57
0:10:57
 0:04:47
0:04:47
 0:04:56
0:04:56
 0:09:06
0:09:06
 0:16:33
0:16:33
 0:14:54
0:14:54
 0:05:15
0:05:15
 0:01:46
0:01:46
 0:41:14
0:41:14
 0:02:46
0:02:46
 0:06:16
0:06:16
 0:36:56
0:36:56
 0:04:28
0:04:28
 0:03:00
0:03:00