filmov
tv
Quantum Chemistry 3.5 - Particle in a Box

Показать описание
Short lecture on particle in a box wavefunctions and energies.
The particle in a box is a model system for a particle which is constrained to a finite region of space. The potential energy is zero inside the box (zero to L) and infinite outside the box. We substitute this potential energy function into the Schrodinger equation and solve for the wavefunction and the energy levels of the particle in a box. Using the boundary conditions that the wavefunction must be zero at the edges of the box, we determine that the wavefunctions are a half integer of a sine wave inside the box. The energy depends quadratically on the positive quantum number n (n = 1, 2, 3), inverse with mass, and inverse quadratic with the length of the box.
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
The particle in a box is a model system for a particle which is constrained to a finite region of space. The potential energy is zero inside the box (zero to L) and infinite outside the box. We substitute this potential energy function into the Schrodinger equation and solve for the wavefunction and the energy levels of the particle in a box. Using the boundary conditions that the wavefunction must be zero at the edges of the box, we determine that the wavefunctions are a half integer of a sine wave inside the box. The energy depends quadratically on the positive quantum number n (n = 1, 2, 3), inverse with mass, and inverse quadratic with the length of the box.
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
Комментарии
 0:07:59
0:07:59
 0:08:42
0:08:42
 0:12:00
0:12:00
 0:14:45
0:14:45
 0:13:39
0:13:39
 0:01:22
0:01:22
 0:05:35
0:05:35
 0:03:32
0:03:32
 0:04:50
0:04:50
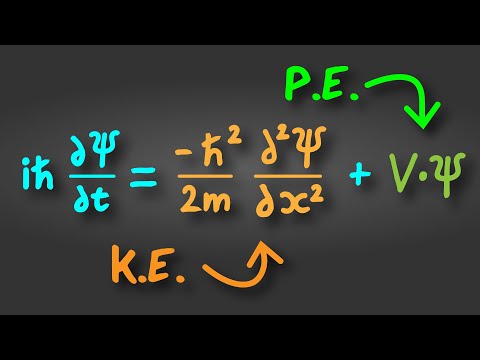 0:01:00
0:01:00
 0:09:44
0:09:44
 0:08:49
0:08:49
 0:05:39
0:05:39
 0:06:30
0:06:30
 0:00:48
0:00:48
 0:00:59
0:00:59
 0:07:11
0:07:11
 0:04:57
0:04:57
 0:47:54
0:47:54
 0:21:44
0:21:44
 0:58:34
0:58:34
 0:16:35
0:16:35
 0:02:02
0:02:02
 0:13:38
0:13:38