filmov
tv
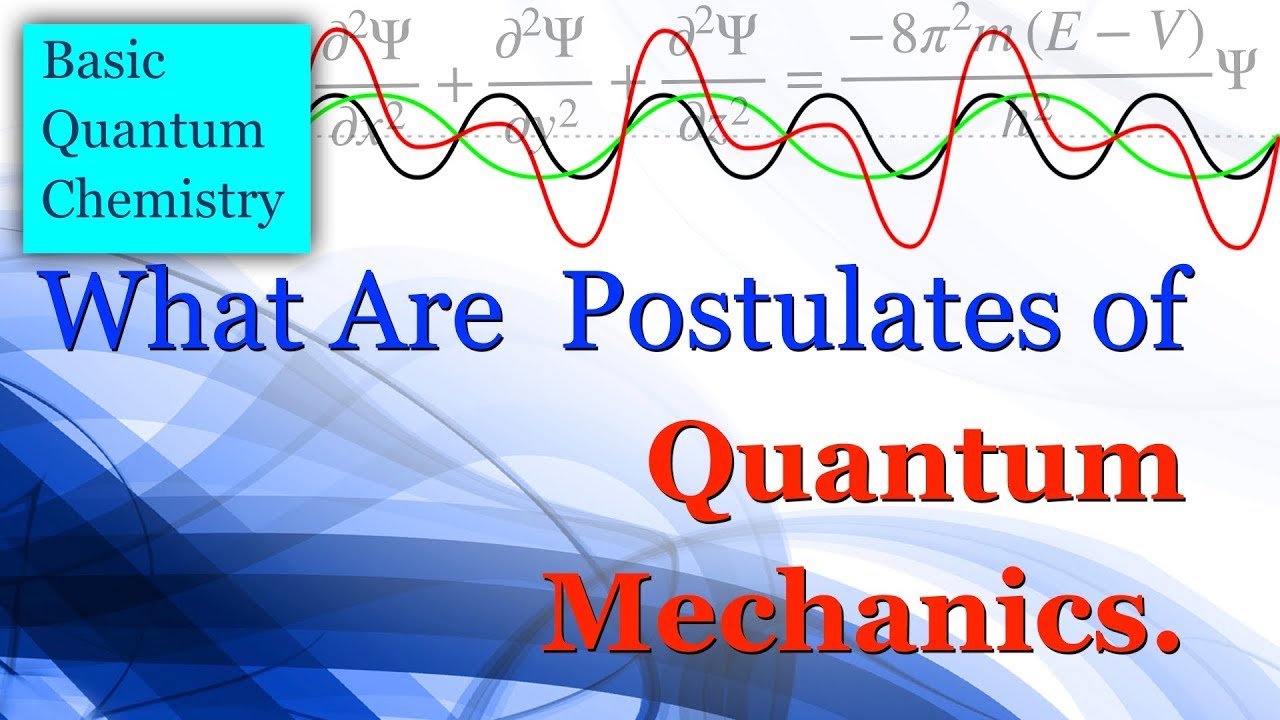
What are the Postulates of Quantum Mechanics - Basic Quantum Chemistry
Показать описание
The relationship between quantum mechanics and operators has helped to present the concepts in the form of some postulates. These Postulates are:
1. The state of a system is described by a wave function 𝚿(x, y, z, t).
2. Every Observable physical property of a system can be characterized by a linear operator. e.g. Position (A) is characterized by operator Â.
3. The possible value of any physical quantity of a system is given by Eigenvalue (a) in the operator equation.
4. When an operator  is operated on a function 𝚿, an average result obtained is given by,
5. As all the wave functions are time-dependent. i.e. 𝚿(x, y, z, t) then its subsequent behaviour should be described by time-dependent Schrödinger equation. Which is,
Where Ĥ is known as Hamiltonian Operator of the system.
Analytical Reasoning
English Grammar
Interview Skills
Managerial Economics
Royalty Free Stock Footage
Chemical Thermodynamics - Physical Chemistry
Ionic Equilibria - Physical Chemistry
Electrochemistry - Physical Chemistry
Solid State - Physical Chemistry
Gaseous State - Physical Chemistry
Colloidal States - Physical Chemistry
Stereochemistry - Organic Chemistry
Nanomaterials - Engineering Chemistry
Water and Its Treatment - Engineering Chemistry
Electrochemistry - Engineering Chemistry
Environmental Studies
Optics - Applied Physics
For Details Visit
1. The state of a system is described by a wave function 𝚿(x, y, z, t).
2. Every Observable physical property of a system can be characterized by a linear operator. e.g. Position (A) is characterized by operator Â.
3. The possible value of any physical quantity of a system is given by Eigenvalue (a) in the operator equation.
4. When an operator  is operated on a function 𝚿, an average result obtained is given by,
5. As all the wave functions are time-dependent. i.e. 𝚿(x, y, z, t) then its subsequent behaviour should be described by time-dependent Schrödinger equation. Which is,
Where Ĥ is known as Hamiltonian Operator of the system.
Analytical Reasoning
English Grammar
Interview Skills
Managerial Economics
Royalty Free Stock Footage
Chemical Thermodynamics - Physical Chemistry
Ionic Equilibria - Physical Chemistry
Electrochemistry - Physical Chemistry
Solid State - Physical Chemistry
Gaseous State - Physical Chemistry
Colloidal States - Physical Chemistry
Stereochemistry - Organic Chemistry
Nanomaterials - Engineering Chemistry
Water and Its Treatment - Engineering Chemistry
Electrochemistry - Engineering Chemistry
Environmental Studies
Optics - Applied Physics
For Details Visit
Комментарии
 0:02:02
0:02:02
 0:02:42
0:02:42
 0:00:22
0:00:22
 0:11:52
0:11:52
 0:01:45
0:01:45
 0:04:57
0:04:57
 0:06:16
0:06:16
 0:05:42
0:05:42
 0:12:52
0:12:52
 0:05:57
0:05:57
 0:07:45
0:07:45
 0:09:22
0:09:22
 0:01:00
0:01:00
 0:02:39
0:02:39
 0:00:43
0:00:43
 0:09:01
0:09:01
 0:00:58
0:00:58
 0:02:37
0:02:37
 0:06:04
0:06:04
 0:18:39
0:18:39
 0:05:50
0:05:50
 0:00:59
0:00:59
 0:00:16
0:00:16
 0:02:36
0:02:36