filmov
tv
Galvanic Cell vs Electrolytic Cell animation| Electrochemical Cells Electrochemistry
Показать описание
In this video we will discuss the galvanic cell vs electrolytic cell animation,similarities and the differences between the Galvanic cell and Electrolytic cell .
Galvanic Cell vs Electrolytic Cell is an important topic of electrochemistry
Similarities between galvanic cell and electrolytic cell:
Both are electrochemical cells
Redox reaction takes place in both cells
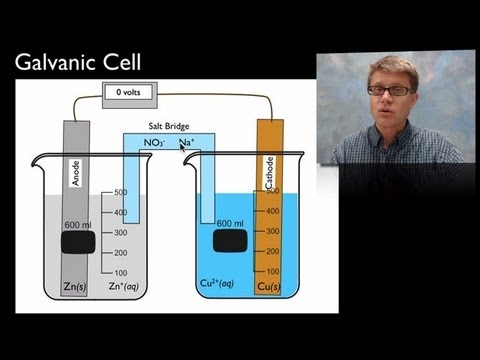
Both cells contain Anode & Cathode
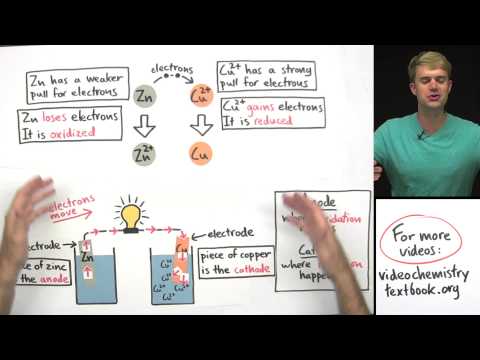
Oxidation takes place at Anode & reduction at Cathode.
Difference b/w Galvanic Cell & Electrolytic Cell:
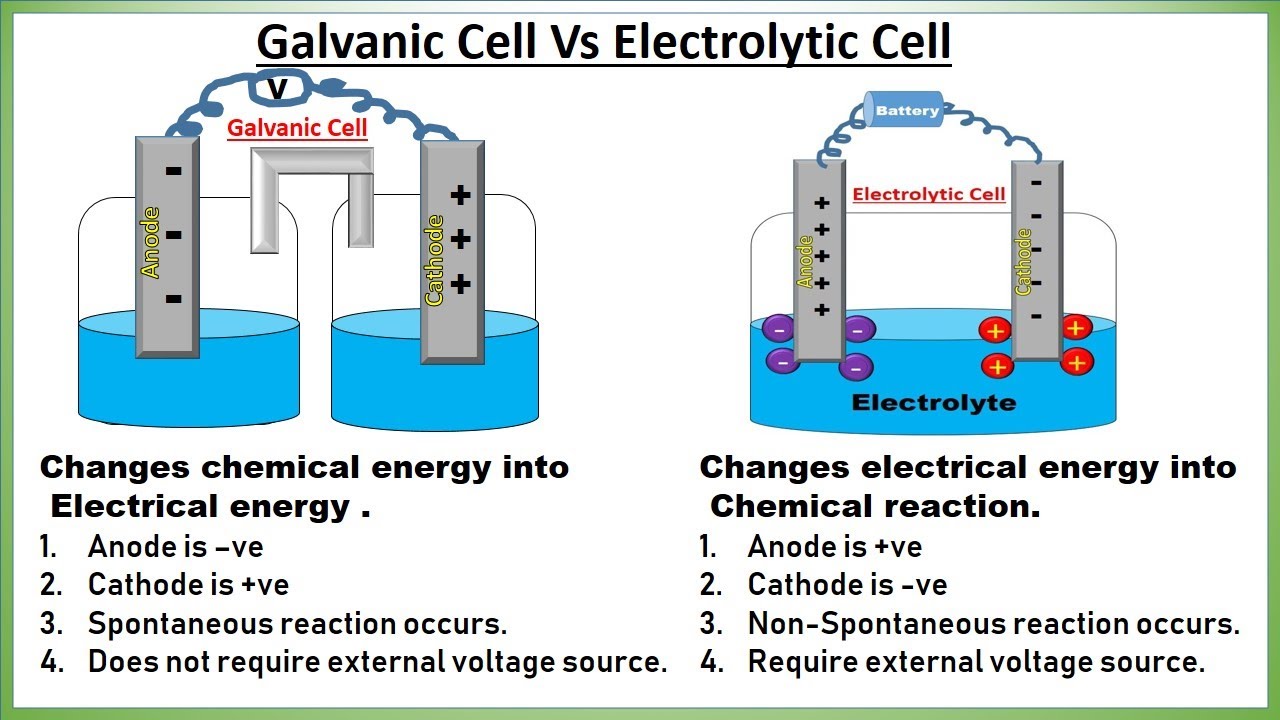
Galvanic cell changes chemical energy into Electrical energy .
Anode is –ve
Cathode is +ve
Spontaneous reaction occurs.
Does not require external voltage source
Electrolytic cell Changes electrical energy into Chemical reaction.
Anode is +ve
Cathode is -ve
Non-Spontaneous reaction occurs.
Require external voltage source.
-----------------------------------------------
👉For joining best Chemistry Coaching, Whatsapp number: 03336753424
Location Islamabad, Pakistan
Free best Chemistry Notes,
Subscribe
Facebook
Instagram
🔴⚫️⚪️🔴⚫️⚪️🔴⚫️⚪️🔴⚫️⚪️🔴⚫️
✅Buy Now !!
✔Online Original Branded Products in Pakistan at the Lowest Rates
✅WhatsApp 03496967013
🔴⚫️⚪️🔴⚫️⚪️🔴⚫️⚪️🔴⚫️⚪️🔴⚫️
For more Chemistry videos:
#digitalkemistry
#DifferenceBetweenGalvanicandElectrolyticCell
#YouTue2021BestChemistryTeacher
#youtube2023
#electrochemistryclass12
#chemistrylecture2023
Galvanic Cell vs Electrolytic Cell is an important topic of electrochemistry
Similarities between galvanic cell and electrolytic cell:
Both are electrochemical cells
Redox reaction takes place in both cells
Both cells contain Anode & Cathode
Oxidation takes place at Anode & reduction at Cathode.
Difference b/w Galvanic Cell & Electrolytic Cell:
Galvanic cell changes chemical energy into Electrical energy .
Anode is –ve
Cathode is +ve
Spontaneous reaction occurs.
Does not require external voltage source
Electrolytic cell Changes electrical energy into Chemical reaction.
Anode is +ve
Cathode is -ve
Non-Spontaneous reaction occurs.
Require external voltage source.
-----------------------------------------------
👉For joining best Chemistry Coaching, Whatsapp number: 03336753424
Location Islamabad, Pakistan
Free best Chemistry Notes,
Subscribe
🔴⚫️⚪️🔴⚫️⚪️🔴⚫️⚪️🔴⚫️⚪️🔴⚫️
✅Buy Now !!
✔Online Original Branded Products in Pakistan at the Lowest Rates
✅WhatsApp 03496967013
🔴⚫️⚪️🔴⚫️⚪️🔴⚫️⚪️🔴⚫️⚪️🔴⚫️
For more Chemistry videos:
#digitalkemistry
#DifferenceBetweenGalvanicandElectrolyticCell
#YouTue2021BestChemistryTeacher
#youtube2023
#electrochemistryclass12
#chemistrylecture2023
Комментарии
 0:02:33
0:02:33
 0:06:55
0:06:55
 0:02:26
0:02:26
 0:13:00
0:13:00
 0:12:59
0:12:59
 0:04:10
0:04:10
 0:27:42
0:27:42
 0:23:35
0:23:35
 0:57:50
0:57:50
 0:06:21
0:06:21
 0:09:04
0:09:04
 0:15:32
0:15:32
 0:02:08
0:02:08
 0:03:41
0:03:41
 0:07:12
0:07:12
 0:04:06
0:04:06
 0:08:44
0:08:44
 0:04:56
0:04:56
 0:11:17
0:11:17
 0:24:18
0:24:18
 0:10:56
0:10:56
 0:14:57
0:14:57
 0:16:37
0:16:37
 0:56:52
0:56:52